Efficient Photocatalytic Two-Electron Halide Oxidation over p-Block Metal Bi- and Sb-Based Catalysts
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
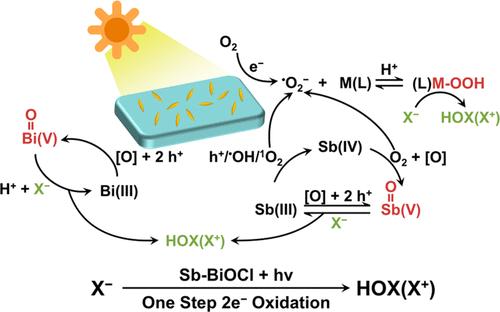
Halide (X–, X = Cl, Br) oxidation provides a promising alternative to water oxidation reaction (WOR) due to the feasibility of the reaction, richness of the reactants, and extensive application of the products (X2, HOX, and OX–) for photocatalysis. Bismuth oxyhalide (BiOX) is still the most efficient photocatalyst applied for halide oxidation ever reported, but relative reaction mechanisms remain understudied, and the reactivity needs to be optimized. Herein, Sb(III) is introduced into BiOX (Sb-BiOX), and by involving itself in the cycle of reactive oxygen species, the reaction was significantly boosted, affording HClO up to 482.5 μM within 30 min. Endowed by the favorable 2e– redox couple due to their unique s2 electron configuration of Bi(III) and Sb(III), the oxidation of X– was performed in a 2e– manner over Sb-BiOX. Because of the introduction of Sb(III), high-valent metal (M(V), M = Sb, Bi) and metal peroxide (M-OOH) could be generated more expeditiously and more easily in situ under light irradiation, which are revealed to be the key oxidizing species for halide oxidation. This work provides an in-depth understanding of promoting the photocatalytic oxidation of halide and could offer more inspiring photocatalytic paradigms for energy-related small-molecule conversion reactions that rely on the WOR half-reaction.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


