Associations of Short-Term Ozone Exposure With Hypoxia and Arterial Stiffness
IF 21.7
1区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
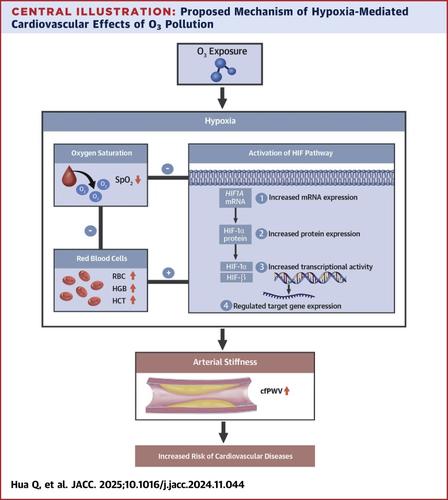
Epidemiological studies reported associations between ozone (O3) exposure and cardiovascular diseases, yet the biological mechanisms remain underexplored. Hypoxia is a shared pathogenesis of O3-associated diseases; therefore, we hypothesized that O3 exposure may induce changes in hypoxia-related markers, leading to adverse cardiovascular effects.
Objectives
This study aimed to investigate associations of short-term O3 exposure with hypoxic biomarkers and arterial stiffness.
Methods
We conducted a panel study involving 210 young healthy residents in 2 cities at different altitudes on the Qinghai-Tibetan Plateau in China, where O3 concentrations are high and particulate pollution is low. Participants underwent 4 repeated visits to assess ambient O3 exposure levels, hypoxic biomarkers, and arterial stiffness. We applied linear mixed-effects models to assess the associations of O3 exposure (lag1 to lag1-7 days) with hypoxic biomarkers and arterial stiffness, adjusted for confounders. Mediation analyses explored the hypoxia’s role in O3-related arterial stiffness changes. We further examined effect modification by residence altitude and the robustness of results by including PM2.5 (particulate matter ≤2.5 μm in aerodynamic diameter) or NO2 in 2-pollutant models.
Results
O3 exposure 1 to 7 days before visits was significantly associated with changes in multiple hypoxic biomarkers. A 10-ppb increase in O3 exposure was linked to significant decreases in oxygen saturation (SpO2) and increases in red blood cell count (RBC), hemoglobin concentration, and hematocrit, with maximum changes by −0.42%, 0.92%, 0.97%, and 1.92%, respectively. Laboratory analysis of mRNA and protein markers consistently indicated that O3 exposure activated the hypoxia-inducible factor 1 (HIF-1) signaling pathway. Additionally, a 10-ppb increase in O3 corresponded to a 1.04% to 1.33% increase in carotid-femoral pulse wave velocity (cfPWV), indicating increased arterial stiffness. RBC, hemoglobin concentration, and hematocrit increases significantly mediated the O3–cfPWV association, whereas the SpO2 reduction had an insignificant mediating effect. Associations of O3 with hypoxic biomarkers varied by altitude. The higher altitude group showed delayed associations with SpO₂ and HIF-1 expression but stronger associations with RBC indices. These associations remained robust after adjusting for copollutants.
Conclusions
O3 exposure may reduce oxygen availability, prompting compensatory increases in red blood cells and hemoglobin, which exacerbate arterial stiffening. These findings provide new insights into the mechanisms underlying O3-induced cardiovascular injury.
短期臭氧暴露与缺氧和动脉硬化的关系
流行病学研究报告了臭氧(O3)暴露与心血管疾病之间的关联,但其生物学机制仍未得到充分探讨。缺氧是氧相关疾病的共同发病机制;因此,我们假设O3暴露可能会引起缺氧相关标志物的变化,从而导致心血管不良反应。目的:本研究旨在探讨短期臭氧暴露与缺氧生物标志物和动脉硬度的关系。方法对中国青藏高原2个不同海拔、臭氧浓度高、颗粒物污染低的城市的210名年轻健康居民进行了小组研究。参与者进行了4次重复访问,以评估环境O3暴露水平、缺氧生物标志物和动脉僵硬度。我们应用线性混合效应模型来评估O3暴露(lag1至lag1-7天)与缺氧生物标志物和动脉硬度的关系,并对混杂因素进行了调整。调解分析探讨缺氧在动脉血氧相关的动脉硬度变化中的作用。通过将PM2.5(空气动力学直径≤2.5 μm的颗粒物)或NO2纳入2种污染物模型,我们进一步检验了居住地海拔对影响的修正以及结果的稳健性。结果访诊前1 ~ 7天so3暴露与多种缺氧生物标志物的变化显著相关。臭氧暴露增加10 ppb与氧饱和度(SpO2)显著降低、红细胞计数(RBC)、血红蛋白浓度和红细胞压积增加有关,最大变化分别为- 0.42%、0.92%、0.97%和1.92%。mRNA和蛋白标记物的实验室分析一致表明,O3暴露激活了缺氧诱导因子1 (HIF-1)信号通路。此外,O3每增加10 ppb,颈-股脉波速度(cfPWV)增加1.04%至1.33%,表明动脉僵硬度增加。红细胞、血红蛋白浓度和红细胞压积的增加显著介导了O3-cfPWV的关联,而SpO2的减少则没有显著的中介作用。O3与缺氧生物标志物的相关性因海拔而异。高海拔组与SpO₂和HIF-1表达的相关性延迟,但与RBC指数的相关性较强。在对共污染物进行调整后,这些关联仍然很强。结论so3暴露可降低氧可用性,促使红细胞和血红蛋白代偿性增加,加重动脉硬化。这些发现为o3诱导心血管损伤的机制提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
42.70
自引率
3.30%
发文量
5097
审稿时长
2-4 weeks
期刊介绍:
The Journal of the American College of Cardiology (JACC) publishes peer-reviewed articles highlighting all aspects of cardiovascular disease, including original clinical studies, experimental investigations with clear clinical relevance, state-of-the-art papers and viewpoints.
Content Profile:
-Original Investigations
-JACC State-of-the-Art Reviews
-JACC Review Topics of the Week
-Guidelines & Clinical Documents
-JACC Guideline Comparisons
-JACC Scientific Expert Panels
-Cardiovascular Medicine & Society
-Editorial Comments (accompanying every Original Investigation)
-Research Letters
-Fellows-in-Training/Early Career Professional Pages
-Editor’s Pages from the Editor-in-Chief or other invited thought leaders

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


