Microbiota translocation following intestinal barrier disruption promotes Mincle-mediated training of myeloid progenitors in the bone marrow
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Impairment of the intestinal barrier allows the systemic translocation of commensal bacteria, inducing a proinflammatory state in the host. Here, we investigated innate immune responses following increased gut permeability upon administration of dextran sulfate sodium (DSS) in mice. We found that Enterococcus faecalis translocated to the bone marrow following DSS treatment and induced trained immunity (TI) hallmarks in bone-marrow-derived mouse macrophages and human monocytes. DSS treatment or heat-killed E. faecalis reprogrammed bone marrow progenitors (BMPs), resulting in enhanced inflammatory responses in vitro and in vivo and protection against subsequent pathogen infections. The C-type lectin receptor Mincle (Clec4e) was essential for E. faecalis-induced TI in BMPs. Clec4e−/− mice showed impaired TI upon E. faecalis administration and reduced pathology following DSS treatment. Thus, Mincle sensing of E. faecalis induces TI that may have long-term effects on pathologies associated with increased gut permeability.
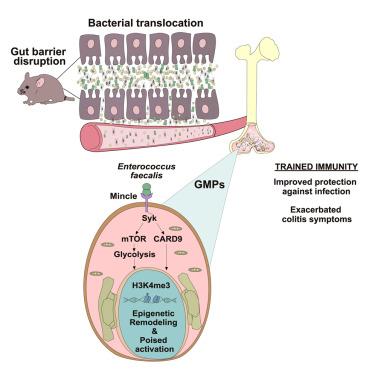
肠道屏障破坏后的微生物群易位促进了骨髓中髓系祖细胞的微颗粒介导的训练
肠道屏障的损伤允许共生菌的全身性易位,在宿主体内诱导促炎状态。在这里,我们研究了小鼠在给予葡聚糖硫酸钠(DSS)后肠道通透性增加后的先天免疫反应。我们发现粪肠球菌在DSS治疗后易位到骨髓,并在骨髓来源的小鼠巨噬细胞和人单核细胞中诱导训练免疫(TI)标志。DSS处理或热杀粪肠球菌重编程骨髓祖细胞(BMPs),导致体外和体内炎症反应增强,并对随后的病原体感染提供保护。c型凝集素受体微环(Clec4e)在BMPs中对粪肠杆菌诱导的TI至关重要。cle4e - / -小鼠在粪肠杆菌治疗后TI受损,DSS治疗后病理减少。因此,粪肠杆菌的Mincle感应诱导TI可能对肠道通透性增加相关的病理有长期影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


