An Endoplasmic Reticulum and Lipid Droplets Dual-localized Strategy to Develop Small Molecular Photosensitizers that Induce Ferroptosis during Photodynamic Therapy
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Organelle-localized photosensitizers have been well-developed to enhance the photodynamic therapy (PDT) efficacy through triggering given cell death. The endoplasmic reticulum (ER) and lipid droplets (LDs) are two key organelles mutually regulating ferroptosis. Thus, in this study, small molecular photosensitizer CAR PSs were developed through fragment integration strategy and the heavy-atom modification. It was showed that the integration strategy did not affect the organelle localization and CAR PSs successfully achieved ER/LDs dual location. Besides, the heavy-atom modification help CAR PSs display good ROS generation efficiency. Importantly, ER/LDs dual-localized CAR PSs exhibited superior photo-toxicity and lower dark-toxicity against multiple breast cancer cell lines than the only ER-targeting Ce6, which further explained the superposition effect of dual organelle targeting. Preliminary studies revealed that CAR PSs induced enhanced ferroptosis via simultaneously triggering the ER stress and lipid peroxidation during PDT. Moreover, CAR-2 demonstrated significant in vivo PDT activity to suppress the tumor growth in 4T1 tumor bearing mice. These findings not only provide a promising photosensitizer CAR-2 exerting excellent in vitro and in vivo PDT effect through stimulating ferroptosis, but also propose a design strategy for the development of ER/LDs dual localized PSs.
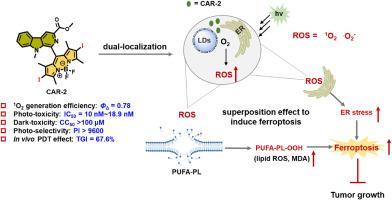
内质网和脂滴双定位策略开发小分子光敏剂,在光动力治疗中诱导铁下垂
细胞器定位光敏剂通过触发给定细胞死亡来增强光动力治疗(PDT)的疗效。内质网(ER)和脂滴(ld)是相互调节铁下垂的两个关键细胞器。因此,本研究通过片段整合策略和重原子修饰,开发了小分子光敏剂CAR - ps。结果表明,整合策略不影响细胞器定位,CAR - ps成功实现了ER/ ld双定位。此外,重原子修饰使CAR - ps具有良好的ROS生成效率。重要的是,与仅靶向ER/LDs的Ce6相比,ER/LDs双定位CAR - ps对多种乳腺癌细胞系表现出更强的光毒性和更低的暗毒性,这进一步解释了双细胞器靶向的叠加效应。初步研究表明,在PDT过程中,CAR - PSs通过同时触发内质网应激和脂质过氧化诱导铁下沉。此外,CAR-2在体内表现出显著的PDT活性,可以抑制4T1荷瘤小鼠的肿瘤生长。这些发现不仅提供了一种很有前景的光敏剂CAR-2,通过刺激铁凋亡在体外和体内发挥出色的PDT作用,而且为ER/LDs双定位PDT的开发提供了设计策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


