MgO Doping on the Sintering and Carbonation Properties of Ternesite
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
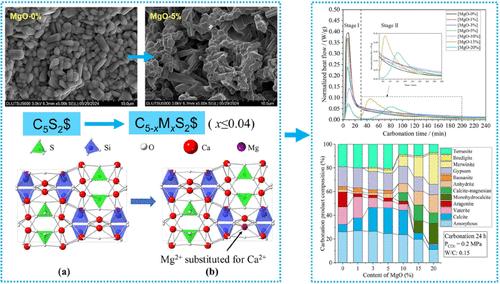
Ternesite has been proven to have significant competitiveness as an ultralow lime CO2 sequestration binder. It is worthy of industrial production for CO2 emission reduction in the cement industry. MgO is inevitable in natural limestone, which may change ternesite’s sintering and carbonation properties. This study aims to simulate the effect of MgO content on the sintering and carbonation behavior of ternesite. The results show that less than 4% Mg2+ is dissolved in the crystal structure of ternesite by replacing Ca2+ and induces a reduction of cell size. More than 4% MgO will be sintered to form bredigite and merwinite, restraining ternesite content in clinkers. The compressive strength of ternesite clinker compacts is negatively correlated with the MgO doping content. The increase in MgO doping from 0 to 20% resulted in a 68.2% decrease in compressive strength. MgO doping less than or equal to 3% improves the CO2 sequestration capacity of ternesite clinkers by 4.1%; however, more than or equal to 5% will reduce the CO2 sequestration capacity. The analysis of carbonation products showed that MgO reduced the content of aragonite and vaterite and induced the formation of magnesian calcite and monohydrocalcite. The difference in ternesite content, crystal morphology, and carbonation products is the reason for the change in carbonation properties of MgO-doped clinkers.
MgO掺杂对特氏体烧结和碳化性能的影响
特立石作为一种超低钙固碳粘合剂已被证明具有显著的竞争力。水泥行业二氧化碳减排值得工业化生产。天然石灰石中不可避免地存在氧化镁,氧化镁会改变钙镁石的烧结和碳化性能。本研究旨在模拟MgO含量对镁铁石烧结和碳化行为的影响。结果表明,小于4%的Mg2+取代Ca2+溶解在钛钙石的晶体结构中,导致细胞尺寸减小。超过4%的MgO将被烧结形成白铝石和镁云石,抑制了熟料中镁云石的含量。镁铁石熟料的抗压强度与MgO掺杂量呈负相关。当MgO掺杂量从0增加到20%时,材料的抗压强度降低了68.2%。MgO掺量小于或等于3%时,固碳能力提高4.1%;但是,超过或等于5%将降低二氧化碳的固存能力。碳化产物分析表明,MgO降低了文石和水晶石的含量,诱发了镁方解石和单水方解石的形成。稀土矿含量、晶体形态和碳化产物的差异是导致掺氧化镁熟料碳化性能变化的原因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


