In situ electrochemical regeneration of permanganate ion for sustainable oxidation reactions
IF 38.6
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Numerous stoichiometric oxidants have been employed for the oxidative production of high-end fine chemicals. However, regeneration of these oxidants often suffers from high energy consumption and complex separation. Here, we report an in situ electrochemical approach for the regeneration of a widely used oxidant, permanganate (MnO4−), by coupling electrochemical and chemical reactions in an integrated system. Using electrosynthesis of 1,3,2-dioxathiolane 2,2-dioxide (DTD), a commercial electrolyte additive in Li-ion batteries, as a representative example, the electrochemically generated MnO4− shows remarkable performance as a redox mediator for catalyzing ethylene sulfite oxidation to produce DTD. By employing pulsed voltammetry, electrosynthesis of DTD can be performed in a single-pass continuous flow electrolyzer with 85% yield and 72% Faradaic efficiency. The practicality of the developed method is demonstrated with a wide substrate scope, robust anode stability, and scaling capability to achieve a DTD production capacity of 500 g/h in a 1-m2 electrolyzer.
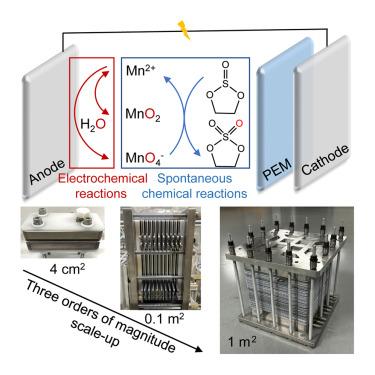

高锰酸盐离子持续氧化反应的原位电化学再生
许多化学计量氧化剂已被用于高端精细化学品的氧化生产。然而,这些氧化剂的再生往往存在高能耗和复杂的分离问题。在这里,我们报道了一种原位电化学方法,通过在一个集成系统中耦合电化学和化学反应来再生广泛使用的氧化剂高锰酸盐(MnO4−)。以锂离子电池电解液添加剂1,3,2-二硫代硫烷2,2-二氧化物(DTD)的电合成为例,电化学生成的MnO4−作为催化亚硫酸乙酯氧化生成DTD的氧化还原介质表现出优异的性能。利用脉冲伏安法,可以在单次连续流电解槽中进行DTD的电合成,收率为85%,法拉第效率为72%。该方法具有广泛的衬底范围、强大的阳极稳定性和缩放能力,可以在1平方米的电解槽中实现500 g/h的DTD生产能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Joule
Energy-General Energy
CiteScore
53.10
自引率
2.00%
发文量
198
期刊介绍:
Joule is a sister journal to Cell that focuses on research, analysis, and ideas related to sustainable energy. It aims to address the global challenge of the need for more sustainable energy solutions. Joule is a forward-looking journal that bridges disciplines and scales of energy research. It connects researchers and analysts working on scientific, technical, economic, policy, and social challenges related to sustainable energy. The journal covers a wide range of energy research, from fundamental laboratory studies on energy conversion and storage to global-level analysis. Joule aims to highlight and amplify the implications, challenges, and opportunities of novel energy research for different groups in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


