Undescribed Hitherto Universal Properties of Isotherms of Excess Adsorption of Liquids and Gases. Connection of Peculiar Points of an Isotherm with Extremums of Its Curvature
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In the proposed paper, a new result is presented, which is as follows: the point on the equilibrium excess adsorption isotherm at which the rate of increase of concentration of a component in the adsorption phase reaches its mean value is the point at which the curvature of the isotherm takes its extreme value. This regularity also holds in the case of the adsorption of gases. This result is confirmed and valid for isotherms of various types. This in turn allows us to directly find the limiting adsorption of the pure component and then the partial isotherm of absolute adsorption. Thus, this result demonstrates that the curvature of the excess adsorption isotherm holds crucial information about the adsorption system, enabling the calculation of absolute adsorption isotherms and several specific characteristics such as the volume of the adsorption space.
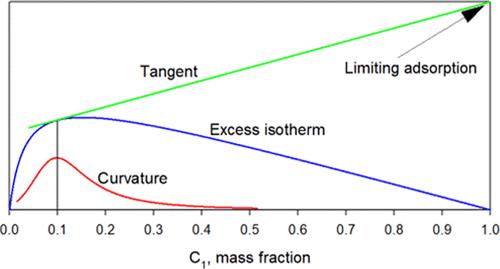
迄今为止所描述的液体和气体过量吸附等温线的普遍特性。等温线奇特点与其曲率极值的联系
本文提出了一个新的结果,即平衡过量吸附等温线上某一组分在吸附相中的浓度增长率达到平均值的点,即等温线曲率达到极值的点。这种规律也适用于气体的吸附。这一结果对各种类型的等温线都是有效的。这反过来又使我们能够直接找到纯组分的极限吸附,然后是绝对吸附的部分等温线。因此,该结果表明,过量吸附等温线的曲率包含有关吸附系统的重要信息,可以计算绝对吸附等温线和吸附空间体积等几个特定特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


