Molecular and cellular morphology of placenta unveils new mechanisms of reproductive immunology
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Despite of numerous studies of the placenta, some molecular and cellular characteristics, particularly the relationship among different cell types, have not been well understood. We aim to investigate the basic and intricate details of cellular and molecular elements in early and late phase placentas to gain better understanding of the immune regulation of human reproductive process.Methods
A novel combination of techniques of spatial transcriptomics(ST), multiple immunohistochemistry, and a dual labeling combining immunohistochemistry and (fluorescence in situ hybridization) FISH on normal and ectopic pregnancy and animal models was employed to investigate the placenta at tissue, cell, protein and molecular levels and to trace the fetal and maternal origin of every cell in early and late placentas.Results
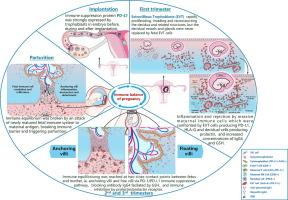
Original discoveries include early expression of immune checkpoint proteins in embryo trophoblasts even before implantation. The detailed distributional relationships among different cell types of fetal and maternal origins in placenta and decidua indicate an immune rejection of the mother towards the fetus and this was counterbalanced by immune inhibitory proteins and blocking antibody Immunoglobulin G4 (IgG4) at the junction between the fetus and the mother. In contrary to common believe, we found that vascular endothelial and glandular epithelial cells in the decidua remain maternal in origin and were not replaced by fetal cells. At term placenta, fetal immune cells infiltrated into the maternal side of the decidus and vice versa indicating a possible immune reaction between fetal and maternal immune systems and suggesting a possible immune mechanism for trigger of parturition. The ability of trophoblasts to create an immune suppressed environment was also supported by findings in ectopic pregnancy and the animal models.Conclusion
The findings indicate a fetus-driven mechanism of immune balance involving both cellular and humoral immunity in human reproduction.
胎盘的分子和细胞形态学揭示了生殖免疫学的新机制
尽管对胎盘进行了大量的研究,但一些分子和细胞特征,特别是不同细胞类型之间的关系,尚未得到很好的理解。我们的目的是研究早期和晚期胎盘细胞和分子元件的基本和复杂的细节,以更好地了解人类生殖过程的免疫调节。方法采用空间转录组学(ST)、多重免疫组织化学技术、免疫组织化学和荧光原位杂交(FISH)双标记技术结合正常妊娠、异位妊娠和动物模型,从组织、细胞、蛋白和分子水平对胎盘进行研究,追踪胎盘早期和晚期各细胞的胎母来源。结果在胚胎着床前,免疫检查点蛋白在胚胎滋养细胞中有早期表达。胎盘和蜕膜中胎儿和母体不同类型细胞之间的详细分布关系表明,母体对胎儿存在免疫排斥,而这种排斥被胎儿和母体交界处的免疫抑制蛋白和阻断抗体免疫球蛋白G4 (IgG4)所抵消。与通常的看法相反,我们发现蜕膜中的血管内皮细胞和腺上皮细胞仍然来自母体,而不是被胎儿细胞所取代。足月胎盘时,胎儿免疫细胞浸润到母体的蜕膜中,反之亦然,这表明胎儿和母体免疫系统之间可能存在免疫反应,可能存在触发分娩的免疫机制。滋养层细胞创造免疫抑制环境的能力也得到异位妊娠和动物模型研究结果的支持。结论胎儿驱动的免疫平衡机制涉及人体生殖过程中的细胞免疫和体液免疫。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
文献相关原料
公司名称
产品信息
索莱宝
BSA
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


