Liquid versus gas-phase operation in heterogeneously catalyzed hydroformylation
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Heterogeneously catalyzed hydroformylation over a commercial 5 % rhodium nanoparticle catalyst has been performed within the intrinsic kinetics regime in a high-throughput kinetics setup. Owing to the use of a paraffinic solvent, the reaction was carried out either in the gas or the liquid phase. An ethylene conversion of around 4 % mol/mol was obtained in the gas-phase, whereas liquid-phase operation allowed achieving around 9 % mol/mol conversion under comparable reaction conditions. The presence of the paraffinic solvent is supposed to better tune the reactant concentration to which the catalyst is exposed. Propanal and ethane were the main products observed, the highest propanal selectivity, 75 % mol/mol, being obtained at the lowest temperature, 120 °C. Apparent activation energies for both hydroformylation (59 kJ/mol (l) / 68 kJ/mol (g)) and hydrogenation (87 kJ/mol (l) / 94 kJ/mol (g)) were found to be lower at liquid compared to gas-phase conditions, suggesting a lower overall surface coverage at liquid-phase conditions. Ethylene and hydrogen were found to exhibit a positive impact on gas-phase hydroformylation and hydrogenation, resulting in higher ethylene conversion when increasing their molar reactant ratios. Based on the positive impact of ethylene observed in the gas-phase operation, it is likely that in the liquid phase, where ethylene solubility is higher, the altered molar ratio distribution at the catalyst surface further enhances yields toward propanal.
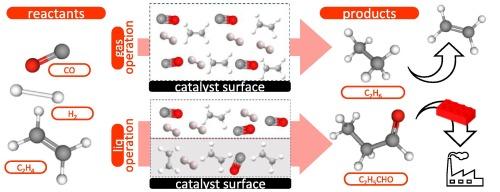
多相催化氢甲酰化的液相和气相操作
在高通量动力学设置中,在商业5 %铑纳米颗粒催化剂上进行了多相催化的氢甲酰化。由于使用了石蜡溶剂,反应可以在气相和液相中进行。在气相中,乙烯转化率约为4 % mol/mol,而在类似的反应条件下,液相操作可实现约9 % mol/mol的转化率。石蜡溶剂的存在被认为可以更好地调节催化剂所暴露的反应物浓度。丙醛和乙烷是主要产物,在最低温度120 ℃时丙醛选择性最高,为75 % mol/mol。氢化甲酰化反应的表观活化能(59 kJ/mol (l) / 68 kJ/mol (g))和氢化反应的表观活化能(87 kJ/mol (l) / 94 kJ/mol (g))在液相条件下比气相条件下更低,表明液相条件下整体表面覆盖率更低。发现乙烯和氢对气相氢甲酰化和加氢反应有积极的影响,当增加它们的摩尔反应物比时,乙烯转化率会更高。根据在气相操作中观察到的乙烯的积极影响,很可能在液相中,乙烯的溶解度更高,催化剂表面摩尔比分布的改变进一步提高了丙醛的产率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


