Preferential CO oxidation (PROX) on LaCoO3–based catalysts: Effect of cobalt oxidation state on selectivity
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The perovskite LaCoO3 (LCO) was used as catalyst for preferential oxidation of CO (PROX). LCO was synthesized via the modified Pechini method and characterized by X-ray diffraction (XRD), high resolution transmission electron microscopy (HRTEM), and CO– and H2– temperature programmed reduction (TPR). Different reductive and oxidative pretreatments were applied to systematically vary the Co oxidation state in order to examine its effect on catalytic performance and to single out active site requirements. Upon reduction at increasing temperature, LaCoO3 transformed to brownmillerite-type La2Co2O5, exsolved Co0 nanoparticles supported on La2O3 and, upon reoxidation, to Co3O4/La2O3. The Co oxidation state of the various catalysts correlated with their CO2 selectivity: LCO containing only Co3+ exhibited 100 % CO2 selectivity in a wide temperature window, whereas La2Co2O5, Co/La2O3 and Co3O4/La2O3 had markedly lower selectivity. It is suggested that Co3+ is crucial and that the strong resistivity of LaCoO3 towards reduction is responsible for the high and stable CO2 selectivity over a temperature range of 100 °C–220 °C. Higher oxygen concentration further broadens the PROX window.

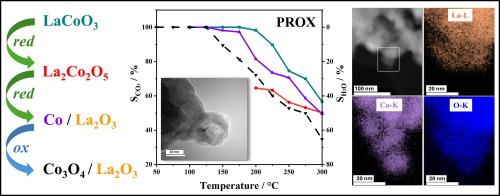
基于lacoo3催化剂的优先CO氧化(PROX):钴氧化态对选择性的影响
以钙钛矿LaCoO3 (LCO)为催化剂,对CO (PROX)进行了优先氧化。采用改进的Pechini法合成了LCO,并通过x射线衍射(XRD)、高分辨率透射电镜(HRTEM)和CO -和H2 -温度程序还原(TPR)对其进行了表征。采用不同的还原和氧化预处理系统地改变Co的氧化状态,以考察其对催化性能的影响,并筛选出活性位点要求。升温还原后,LaCoO3转化为褐煤型La2Co2O5,析出以La2O3为载体的Co0纳米颗粒,再氧化后转化为Co3O4/La2O3。不同催化剂的Co氧化态与其CO2选择性相关:仅含Co3+的LCO在宽温度窗内具有100 %的CO2选择性,而La2Co2O5、Co/La2O3和Co3O4/La2O3的选择性明显较低。这表明Co3+是至关重要的,并且LaCoO3对还原的强电阻率是在100 °C - 220 °C的温度范围内具有高而稳定的CO2选择性的原因。更高的氧浓度进一步拓宽了PROX窗口。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


