Pickering Interfacial Tandem Catalysis of Alkenes to 1,2-Diols over Manganese Oxide Catalysts at Room Temperature
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In the tandem synthesis of 1,2-cyclohexanediol, solvents such as acetonitrile are often added to eliminate the immiscibility of cyclohexene with water and accordingly strengthen the interphase mass transfer; however, the usage of solvents artificially increases the solvent separation procedure, enhancing the energy consumption and decreasing the economic benefits of the reaction process. Hence, the development of the solvent-free Pickering interfacial tandem catalysis of cyclohexene to 1,2-cyclohexanediol is extremely appealing. In this study, β-MnO2 and Mn2O3 were prepared by calcining γ-MnO2 synthesized with the hydrothermal synthesis method and concurrently served as a colloidal emulsifier and a heterogeneous catalyst in the Pickering interfacial tandem catalysis of cyclohexene to 1,2-cyclohexanediol at room temperature in the presence of the oxidant molecular oxygen and the co-oxidant isobutyraldehyde. The prepared β450-MnO2 sample revealed the best tandem catalysis performance, achieving a cyclohexene conversion of 99.4% and a 1,2-cyclohexanediol selectivity of 83.6% within 4 h of reaction, which can be ascribed to the highest Mn4+/Mn3+ ratio and the greatest concentration of oxygen vacancies as well as the most stable Pickering emulsion. At the same time, density functional theory (DFT) studies further confirmed that isobutyraldehyde and molecular oxygen could be more easily adsorbed and activated by β450-MnO2 in comparison with the other catalyst samples, benefiting its eminent catalytic epoxidation performance. In addition, a possible reaction mechanism for the β450-MnO2 catalyst catalyzing cyclohexene into 1,2-cyclohexanediol in the Pickering interfacial tandem catalytic reaction system was put forward and validated through quenching experiments as well as in situ infrared characterization. The synthesized β450-MnO2 catalyst exhibited reusability for greater than 5 cycles, and meanwhile, the Pickering interfacial tandem catalytic reaction system can be expanded to a spread of linear and cyclic alkene substrates, highlighting the superiority of the β450-MnO2 catalyst. These findings verify that the synthesized β450-MnO2 catalyst is capable of being utilized as an efficient and stable catalyst for the Pickering interfacial catalytic conversion of alkenes into 1,2-diols at room temperature.
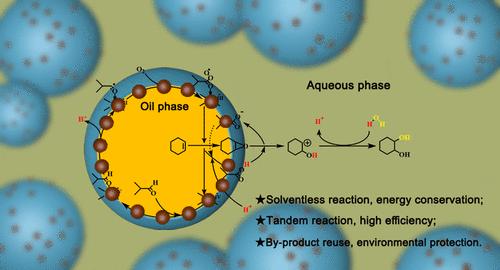
室温下氧化锰催化烯烃制1,2-二醇的Pickering界面串联催化
在串联合成1,2-环己二醇时,常加入乙腈等溶剂以消除环己烯与水的不混相,从而加强相间传质;然而,溶剂的使用人为地增加了溶剂分离过程,增加了能量消耗,降低了反应过程的经济效益。因此,开发无溶剂Pickering界面串联催化环己烯制1,2-环己二醇是非常有吸引力的。本研究将水热法合成的γ-MnO2煅烧制备β-MnO2和Mn2O3,在氧化剂分子氧和助氧化剂异丁醛存在下,同时作为胶体乳化剂和非均相催化剂,在室温下进行了环己烯制1,2-环己二醇的Pickering界面级联催化反应。制备的β450-MnO2样品显示出最佳的级联催化性能,在反应4 h内,环己烯转化率为99.4%,1,2-环己二醇选择性为83.6%,这可得益于最高的Mn4+/Mn3+比和最大的氧空位浓度,以及最稳定的Pickering乳状液。同时,密度泛函理论(DFT)研究进一步证实,与其他催化剂样品相比,β450-MnO2更容易吸附和激活异丁醛和分子氧,从而使其具有优异的催化环氧化性能。此外,提出了β450-MnO2催化剂在Pickering界面级联催化反应体系中催化环己烯生成1,2-环己二醇的可能反应机理,并通过淬火实验和原位红外表征进行了验证。合成的β450-MnO2催化剂具有大于5个循环的可重复使用性,同时,Pickering界面串联催化反应体系可以扩展到线性和环烯烃底物,突出了β450-MnO2催化剂的优越性。这些结果验证了合成的β450-MnO2催化剂能够作为一种高效、稳定的催化剂用于室温下烯烃的Pickering界面催化转化为1,2-二醇。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


