Shear-Stress Initiates Signal Two of NLRP3 Inflammasome Activation in LPS-Primed Macrophages through Piezo1
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The innate immune system is tightly regulated by a complex network of chemical signals triggered by pathogens, cellular damage, and environmental stimuli. While it is well-established that changes in the extracellular environment can significantly influence the immune response to pathogens and damage-associated molecules, there remains a limited understanding of how changes in environmental stimuli specifically impact the activation of the NLRP3 inflammasome, a key component of innate immunity. Here, we demonstrated how shear stress can act as Signal 2 in the NLRP3 inflammasome activation pathway by treating LPS-primed immortalized bone marrow-derived macrophages (iBMDMs) with several physiologically relevant magnitudes of shear stress to induce inflammasome activation. We demonstrated that magnitudes of shear stress within 1.0 to 50 dyn/cm2 were able to induce ASC speck formation, while 50 dyn/cm2 was sufficient to induce significant calcium signaling, gasdermin-D cleavage, caspase-1 activity, and IL-1β secretion, all hallmarks of inflammasome activation. Utilizing NLRP3 and caspase-1 knockout iBMDMs, we demonstrated that the NLRP3 inflammasome was primarily activated as a result of shear stress exposure. Quantitative polymerase chain reaction (qPCR), ELISA, and a small molecule inhibitor study aided us in demonstrating that expression of Piezo1, NLRP3, gasdermin-D, IL-1β, and CCL2 secretion were all upregulated in iBMDMs treated with shear stress. This study provides a foundation for further understanding the interconnected pathogenesis of chronic inflammatory diseases and the ability of shear stress to play a role in their progression.
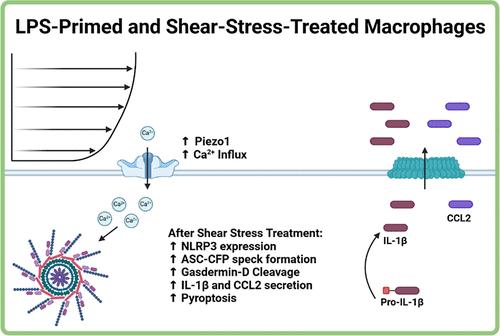
剪切应力通过 Piezo1 触发 LPS 诱导的巨噬细胞中 NLRP3 炎症小体活化的信号二
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


