Construction of chlorine-free electrical double layer for efficient seawater oxidation
IF 20.3
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Seawater electrolysis is an effective means to solve the problems of transport and consumption of offshore wind electricity. The corrosion reaction and chlorine oxidation reaction caused by the existence of chloride in seawater lead to poor electrochemical performances and low efficiencies for seawater oxidation electrocatalysts. Hence, construction of chlorine-free electrical double layer (EDL) has become one hotspot for efficient seawater oxidation. Based on the theories of interfacial electric field, adsorption selectivity, steric hindrance and electrostatic repulsion, six practical strategies have been well developed to construct the chlorine-free EDL, i.e., high-curvature morphology, hard Lewis acid coating, ploy-homoatomic anion, coordinated common ion, oxyanion, and hard Lewis acid with oxyanion. Great progresses have been made in electrocatalyst stability and reaction selectivity. However, five problems still exist limiting the pullulation of seawater oxidation: (i) the constructed EDL not completely chlorine-free. (ii) too simple condition for EDL simulation, (iii) adverse effects of modification components, (iv) lack of influences of adsorbed oxygen-containing intermediates on electrocatalysts, and (v) ignorance of solvated cations and anions in EDL. Finally, five prospects are proposed to propel the developments of chloride-free EDL and seawater electrolysis: (i) combination of multiple strategies for chlorine-free EDL construction, (ii) investigation of dynamical EDL structures by time-responsive information of electrocatalysts and electrolysis interfaces, (iii) precise control of modification components by subtle electrocatalyst preparation and appropriate electrolyte additive, (iv) encompassing oxygen-containing intermediates, and (v) involvement of solvated cations and anions in the simulated EDL. Besides, the understanding of EDL would be deeper, promoting its wide application in the fields of information, energy conversion and storage.

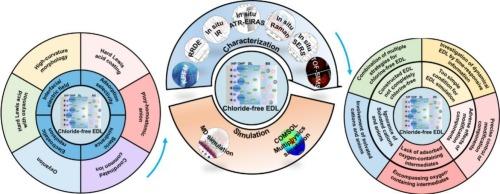
用于海水高效氧化的无氯电双层结构
海水电解是解决海上风电运输和消费问题的有效手段。海水中氯离子的存在引起的腐蚀反应和氯氧化反应导致海水氧化电催化剂的电化学性能差,效率低。因此,构建无氯双电层(EDL)已成为海水高效氧化的研究热点之一。基于界面电场、吸附选择性、位阻和静电斥力等理论,提出了高曲率形貌、硬Lewis酸包覆、聚同原子阴离子、配位普通离子、氧阴离子和硬Lewis酸带氧阴离子6种构建无氯EDL的实用策略。在电催化剂稳定性和反应选择性方面取得了很大进展。然而,目前仍存在五个问题限制了海水氧化的发展:(1)建成的EDL不完全无氯。(ii)模拟EDL的条件过于简单,(iii)改性组分的不利影响,(iv)缺乏吸附的含氧中间体对电催化剂的影响,以及(v)忽略EDL中溶剂化的阳离子和阴离子。最后,提出了推动无氯EDL和海水电解发展的五个前景:(i)结合多种策略构建无氯EDL; (ii)通过电催化剂和电解界面的时间响应信息来研究动态EDL结构;(iii)通过精细的电催化剂制备和适当的电解质添加剂来精确控制修饰成分;(iv)包含含氧中间体;(v)在模拟EDL中参与溶剂化的阳离子和阴离子。此外,人们对EDL的理解也会加深,促进其在信息、能量转换和存储等领域的广泛应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Coordination Chemistry Reviews
化学-无机化学与核化学
CiteScore
34.30
自引率
5.30%
发文量
457
审稿时长
54 days
期刊介绍:
Coordination Chemistry Reviews offers rapid publication of review articles on current and significant topics in coordination chemistry, encompassing organometallic, supramolecular, theoretical, and bioinorganic chemistry. It also covers catalysis, materials chemistry, and metal-organic frameworks from a coordination chemistry perspective. Reviews summarize recent developments or discuss specific techniques, welcoming contributions from both established and emerging researchers.
The journal releases special issues on timely subjects, including those featuring contributions from specific regions or conferences. Occasional full-length book articles are also featured. Additionally, special volumes cover annual reviews of main group chemistry, transition metal group chemistry, and organometallic chemistry. These comprehensive reviews are vital resources for those engaged in coordination chemistry, further establishing Coordination Chemistry Reviews as a hub for insightful surveys in inorganic and physical inorganic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


