Structural mechanism underlying PHO1;H1-mediated phosphate transport in Arabidopsis
IF 13.6
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
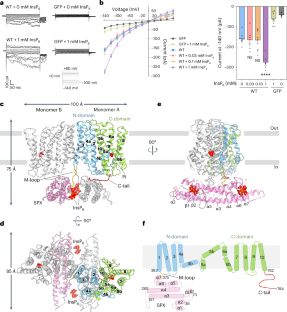
Arabidopsis PHOSPHATE 1 (AtPHO1) and its closest homologue AtPHO1;H1 are phosphate transporters that load phosphate into the xylem vessel for root-to-shoot translocation. AtPHO1 and AtPHO1;H1 are prototypical members of the unique SPX–EXS family, whose structural and molecular mechanisms remain elusive. In this study, we determined the cryogenic electron microscopy structure of AtPHO1;H1 binding with inorganic phosphate (Pi) and inositol hexakisphosphate in a closed conformation. Further molecular dynamic simulation and AlphaFold prediction support an open conformation. AtPHO1;H1 forms a domain-swapped homodimer that involves both the transmembrane ERD1/XPR1/SYG1 (EXS) domain and the cytoplasmic SYG1/Pho81/XPR1 (SPX) domain. The EXS domain presented by the SPX–EXS family represents a novel protein fold, and an independent substrate transport pathway and substrate-binding site are present in each EXS domain. Two gating residues, Trp719 and Tyr610, are identified above the substrate-binding site to control opening and closing of the pathway. The SPX domain features positively charged patches and/or residues at the dimer interface to accommodate inositol hexakisphosphate molecules, whose binding mediates dimerization and enhances AtPHO1;H1 activity. In addition, a C-terminal tail is required for AtPHO1;H1 activity. On the basis of structural and functional analysis, a working model for Pi efflux mediated by AtPHO1;H1 and its homologues was postulated, suggesting a channel-like mechanism. This study not only reveals the molecular and regulatory mechanism underlying Pi transport mediated by the unique SPX–EXS family, but also provides potential for crop engineering to enhance phosphorus-use efficiency in sustainable agriculture. This study characterizes the plant inorganic phosphate transporter PHO1 through cryogenic electron microscopy and biochemical and physiological analysis, revealing the molecular and regulatory mechanism of transport mediated by the SPX–EXS family.

拟南芥PHO1的结构机制;h1介导的磷酸盐运输
拟南芥磷酸1 (AtPHO1)及其最近的同源物AtPHO1;H1是磷酸盐转运蛋白,将磷酸盐装载到木质部导管中进行根到茎的转运。AtPHO1和AtPHO1;H1是独特的SPX-EXS家族的原型成员,其结构和分子机制尚不清楚。在这项研究中,我们确定了AtPHO1的低温电镜结构;H1与无机磷酸盐(Pi)和肌醇六磷酸以封闭的构象结合。进一步的分子动力学模拟和AlphaFold预测支持开放构象。H1形成一个结构域交换的同二聚体,涉及跨膜ERD1/XPR1/SYG1 (EXS)结构域和细胞质SYG1/Pho81/XPR1 (SPX)结构域。SPX-EXS家族提出的EXS结构域代表了一种新的蛋白质折叠,每个EXS结构域都存在独立的底物转运途径和底物结合位点。两个门控残基Trp719和Tyr610位于底物结合位点上方,控制通路的开启和关闭。SPX结构域在二聚体界面处具有带正电的斑块和/或残基,以容纳肌醇己基磷酸分子,其结合介导二聚化并增强AtPHO1;H1活性。此外,AtPHO1 H1活性需要c端尾部。在结构和功能分析的基础上,我们假设了一个由AtPHO1 H1及其同源物介导的Pi外排的工作模型,提出了一个类似通道的机制。该研究不仅揭示了独特的SPX-EXS家族介导磷转运的分子和调控机制,而且为作物工程提高可持续农业中磷的利用效率提供了潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


