Natural sunlight induced photocatalytic hydrogen evolution by SrTaO2N/CdS nanocomposites
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
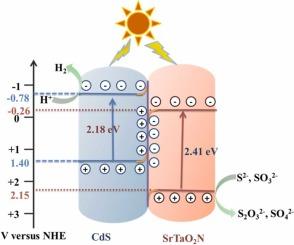
The oxynitride SrTaO2N is a potential semiconductor for converting solar energy into hydrogen energy due to its visible light absorption capability. However, the catalytic activity is low due to fast electron-hole (e--h+) recombination. Here, we have synthesized SrTaO2N/CdS heterojunction by using the co-precipitation method to improve the catalytic activity. SrTaO2N/CdS nanocomposite is studied for photocatalytic H2 evolution in the presence of Na2S/Na2SO3 under both natural sunlight and artificial light (Xe lamp). Among the synthesized nanocomposites, the 5 % SrTaO2N/CdS heterojunction exhibits the highest H2 generation of 40.25 mmol for 5 h under visible light produced by a xenon lamp without utilizing a co-catalyst, and the apparent quantum efficiency of 5 % SrTaO2N/CdS is found to be 11.8 ± 0.1 % at a wavelength of 400 nm. This is much higher than that of the catalytic activity shown by SrTaO2N and CdS independently under similar experimental conditions. It is because of the effective e--h+ pair separation in the nanocomposite due to the Z-scheme heterojunction formation between SrTaO2N and CdS semiconductors with valance, conduction band positions at 2.15, −0.26 eV vs SHE for SrTaO2N and 1.40, −0.78 eV vs SHE for CdS, respectively. This composite exhibits hydrogen evolution activity of 40.04 mmol for 5 h under natural sunlight, which is a promising step towards practical applications.
SrTaO2N/CdS纳米复合材料的光催化析氢研究
氮氧化合物SrTaO2N具有可见光吸收能力,是一种将太阳能转化为氢能的潜在半导体材料。然而,由于电子-空穴(e—h+)快速复合,催化活性较低。本文采用共沉淀法合成了SrTaO2N/CdS异质结,提高了催化活性。研究了SrTaO2N/CdS纳米复合材料在Na2S/Na2SO3存在下,在自然光和人造光(Xe灯)下光催化析氢的性能。在所合成的纳米复合材料中,5% SrTaO2N/CdS异质结在不使用助催化剂的情况下,在氙灯可见光下产生5 h的H2产量最高,为40.25 mmol;在400 nm波长下,5% SrTaO2N/CdS的表观量子效率为11.8±0.1%。这远远高于SrTaO2N和CdS在相似实验条件下单独表现出的催化活性。这是由于SrTaO2N和CdS半导体之间形成的Z-scheme异质结导致纳米复合材料中e—h+对有效分离,SrTaO2N的导带价位分别为2.15、-0.26 eV / SHE和1.40、-0.78 eV / SHE。该复合材料在自然光照下5 h的析氢活性为40.04 mmol,具有较好的实际应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


