Novel tetracycline-degrading enzymes from the gut microbiota of black soldier fly: Discovery, performance, degradation pathways, mechanisms, and application potential
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
The antibiotic tetracycline (TC) is an emerging pollutant frequently detected in various environments. Although enzymatic remediation is a promising strategy for mitigating TC contamination, the availability of effective TC-degrading enzymes remains limited, and their mechanisms and applications are not fully understood. This study developed a comprehensive TC-degrading enzyme library from the gut microbiome of the highly TC-resistant saprophagous insect, black soldier fly larvae (BSFL), using an integrated metagenomic and comparative metatranscriptomic approach, identifying 105 potential novel TC-degradation genes. Bioinformatics analysis of 10 selected genes underscored the novelty of the identified enzymes. Among these, Trg2 demonstrated strong binding affinity and significant degradation capacity for TC. Key functional amino acid residues, including Thr231, Ala64, Ala82, Gly68, Gly79, and Ser81, were identified as essential for the interaction between TC and Trg2. Six TC degradation pathways were proposed, involving the transformation of TC into 19 metabolites through de-grouping, ring opening, oxidation, reduction, and addition reactions, effectively reducing TC toxicity. Furthermore, Trg2 exhibited resilience under harsh conditions, maintaining the capacity to remove about 45 % of the total TC in mariculture wastewater across eight successive batches. This study advances the understanding of TC degradation mechanisms and highlights the potential application of novel enzymes for bioremediation purposes.
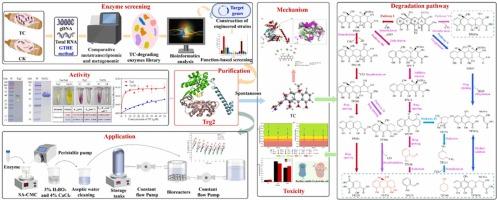
来自黑兵蝇肠道微生物群的新型四环素降解酶:发现、性能、降解途径、机制和应用潜力
抗生素四环素(四环素)是在各种环境中经常检测到的一种新兴污染物。虽然酶修复是减轻TC污染的一种很有前途的策略,但有效的TC降解酶的可用性仍然有限,其机制和应用尚未完全了解。本研究从高度耐tc腐食昆虫黑兵蝇幼虫(BSFL)的肠道微生物群中建立了一个全面的tc降解酶文库,采用综合宏基因组学和比较亚转录组学方法,鉴定了105个潜在的新的tc降解基因。10个选定基因的生物信息学分析强调了所鉴定酶的新颖性。其中,Trg2对TC具有较强的结合亲和力和显著的降解能力。关键的功能氨基酸残基,包括Thr231, Ala64, Ala82, Gly68, Gly79和Ser81,被鉴定为TC和Trg2之间相互作用的必需氨基酸。提出了6条TC降解途径,通过脱基团、开环、氧化、还原和加成反应将TC转化为19种代谢物,有效降低了TC的毒性。此外,Trg2在恶劣条件下表现出弹性,在连续八个批次中保持了去除海水养殖废水中总TC约45%的能力。这项研究促进了对TC降解机制的理解,并强调了新型酶在生物修复中的潜在应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


