Variants and vaccines impact nasal immunity over three waves of SARS-CoV-2
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
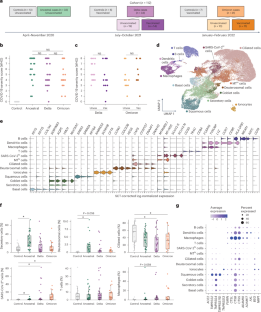
Viral variant and host vaccination status impact infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), yet how these factors shift cellular responses in the human nasal mucosa remains uncharacterized. We performed single-cell RNA sequencing (scRNA-seq) on nasopharyngeal swabs from vaccinated and unvaccinated adults with acute Delta and Omicron SARS-CoV-2 infections and integrated with data from acute infections with ancestral SARS-CoV-2. Patients with Delta and Omicron exhibited greater similarity in nasal cell composition driven by myeloid, T cell and SARS-CoV-2hi cell subsets, which was distinct from that of ancestral cases. Delta-infected samples had a marked increase in viral RNA, and a subset of PER2+EGR1+GDF15+ epithelial cells was enriched in SARS-CoV-2 RNA+ cells in all variants. Prior vaccination was associated with increased frequency and activation of nasal macrophages. Expression of interferon-stimulated genes negatively correlated with coronavirus disease 2019 (COVID-19) severity in patients with ancestral and Delta but not Omicron variants. Our study defines nasal cell responses and signatures of disease severity across SARS-CoV-2 variants and vaccination. Ordovas-Montanes and colleagues describe the composition of the nasal cellular ecosystem and signatures of disease severity in vaccinated and unvaccinated adults during infection with the ancestral, Delta and Omicron variants of SARS-CoV-2.

变体和疫苗在三波SARS-CoV-2中影响鼻腔免疫
病毒变异和宿主疫苗接种状况影响严重急性呼吸综合征冠状病毒2型(SARS-CoV-2)感染,但这些因素如何改变人鼻黏膜的细胞反应尚不清楚。我们对接种疫苗和未接种疫苗的急性德尔塔型和欧米克隆型SARS-CoV-2感染成人的鼻咽拭子进行了单细胞RNA测序(scRNA-seq),并整合了祖先型SARS-CoV-2急性感染的数据。Delta和Omicron患者在髓系、T细胞和SARS-CoV-2hi细胞亚群驱动的鼻腔细胞组成上表现出更大的相似性,这与祖先病例不同。在所有变异的SARS-CoV-2 RNA+细胞中,δ感染样本的病毒RNA显著增加,并且一个PER2+EGR1+GDF15+上皮细胞亚群富集。先前的疫苗接种与鼻巨噬细胞的频率和激活增加有关。干扰素刺激基因的表达与祖先型和Delta型而非Omicron型变异患者的2019冠状病毒病(COVID-19)严重程度负相关。我们的研究定义了SARS-CoV-2变体和疫苗接种的鼻细胞反应和疾病严重程度的特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


