Discovery of Novel Fluorine-Containing Parthenolide Analogues as Potential Antitumor Agents
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Incorporating fluorine-containing groups into the chemical skeleton is expected to enhance bioactivity and bioavailability. Directly introducing fluorine groups into the parthenolide skeleton remains challenging and limited. In this research, a series of novel fluorine-containing parthenolide derivatives were synthesized through late-stage diversification strategy. And the most promising derivate 1 exhibited good antiproliferative activity against NCI-H820 (IC50: 2.66 μM), Huh-7 (IC50: 2.36 μM), and PANC-1(IC50: 2.16 μM). The preliminary mechanism study indicated compound 1 strongly inhibited the colony formation number of NCI-H820, Huh-7 and PANC-1 cells and inhibited lung cancer metastasis with a dose-dependent manner through inhibiting STAT3 signaling pathway. Compound 16, a prodrug of compound 1, showed a significant improvement in aqueous solubility and oral bioavailability compared with parthenolide. Moreover, compound 16 significantly suppressed tumor growth in lung patient-derived tumor xenograft model without obvious toxicity. Based on the above results, we propose that compound 16 may be a promising lead compound for treatment of lung cancer.
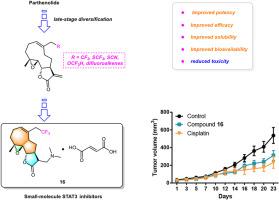
新型含氟Parthenolide类似物作为潜在抗肿瘤药物的发现
在化学骨架中加入含氟基团有望提高生物活性和生物利用度。直接将氟基团引入孤雌醇内酯骨架仍然具有挑战性和局限性。本研究通过后期多样化策略合成了一系列新型含氟孤香内酯衍生物。其中最有希望的衍生物1对NCI-H820 (IC50: 2.66 μM)、Huh-7 (IC50: 2.36 μM)和PANC-1(IC50: 2.16 μM)具有良好的抗增殖活性。初步机制研究表明,化合物1通过抑制STAT3信号通路,强烈抑制NCI-H820、Huh-7和PANC-1细胞集落形成数量,并呈剂量依赖性抑制肺癌转移。化合物16是化合物1的前药,与parthenolide相比,化合物16的水溶性和口服生物利用度有显著改善。此外,化合物16在肺源性肿瘤异种移植模型中明显抑制肿瘤生长,且无明显毒性。基于以上结果,我们认为化合物16可能是治疗肺癌的先导化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


