Engineering the Fungal Peroxygenase for Efficient and Regioselective Hydroxylation of Vitamin Ds and Sterols
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Hydroxylation of C25 C–H bonds (referring to sterols) is of great importance in vivo for metabolizing sterols and vitamin Ds. The biocatalytic hydroxylation of C25 C–H bonds is restricted by the selectivity and activity of the enzymes due to the inertness of these bulky compounds. Herein, we employed fungal unspecific peroxygenase from Agrocybe aegerita (AaeUPO) as the catalyst to develop efficient and selective AaeUPO variants through protein engineering. After three rounds of evolution using semirational design, 2 variants, G195A/G241V/G318V (Stev) and Q72K/G195A/G241V (Veco), were determined to be the ideal catalysts, showing a 25- to 27-fold increase in enzyme activity and an improvement in selectivity from 25% to over 93% in gram-scale conversion of vitamin D3 to 25-hydroxyvitamin D3. These two variants exhibited overall enhanced catalytic performance in hydroxylating the C25 C–H bonds of the other 24 sterol and vitamin D analogues. This work provides an enzymatic toolbox to synthesize the highly important vitamins and sterols into the compounds of interest under mild conditions with remarkable regioselectivity and enzyme activity.
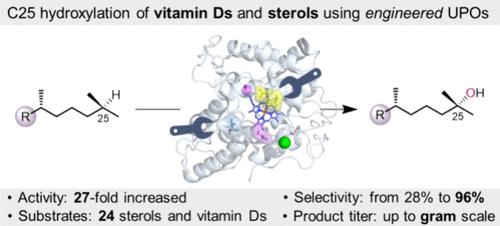
设计真菌过加氧酶高效和区域选择性羟化维生素d和甾醇
C25 C-H键(指固醇)的羟基化对体内代谢固醇和维生素d非常重要。由于这些大体积化合物的惰性,C25 C-H键的生物催化羟基化受到酶的选择性和活性的限制。本研究以来自Agrocybe aegerita的真菌非特异性过氧酶(AaeUPO)为催化剂,通过蛋白质工程技术开发出高效、选择性的AaeUPO变体。经过三轮半设计进化,确定了G195A/G241V/G318V (Stev)和Q72K/G195A/G241V (Veco)两个变体是理想的催化剂,在维生素D3转化为25-羟基维生素D3的克级转化率上,酶活性提高了25- 27倍,选择性从25%提高到93%以上。这两种变体在羟基化其他24种甾醇和维生素D类似物的C25 C-H键方面表现出整体增强的催化性能。这项工作提供了一个酶工具箱,在温和的条件下合成高度重要的维生素和甾醇为感兴趣的化合物,具有显著的区域选择性和酶活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


