Probing the Interaction Mechanisms of Dodecane and Mica/Calcite: Implications for Oil Recovery
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Understanding the interfacial interaction mechanisms between oil and minerals is of vital importance in the applications of petroleum production and environmental protection. In this work, the interactions of dodecane with mica and calcite in aqueous media were investigated by using the drop probe technique based on atomic force microscopy. For the dodecane–mica interactions, the electrical double layer (EDL) repulsion dominated in 10 mM NaCl solution, and a higher pH facilitated the detachment of dodecane. The EDL interaction was diminished with an elevated NaCl concentration, and the van der Waals (vdW) attraction triggered the oil attachment on the mica surface. In the presence of Ca2+ and Mg2+, the EDL repulsion was weakened leading to a thinner water film, and the oil attachment occurred. For the dodecane–calcite interactions, large adhesions were observed under all of the solution conditions, mainly attributed to the ionic bridging by Ca2+ formed during calcite dissolution. At pH 4, CO2 bubbles appeared on the calcite surface, resulting in a repulsive vdW interaction between dodecane and the surface, which hindered the adhesion. The calcite surface wettability was modified in high concentrations of NaCl, leading to a reduced adhesion with oil. The ionic bridging effect was promoted, and the adhesion was enhanced in 500 mM Ca2+ and Mg2+ solutions. At pH 10, the precipitates of Ca(OH)2(s) and Mg(OH)2(s) appeared on the calcite surface, which diminished the adhesion. Our results provided fundamental insights into the mechanisms of oil–water–mineral interactions, which contributed to the understanding and modulation of engineering processes in the enhancement of oil pollutant removal and oil recovery.
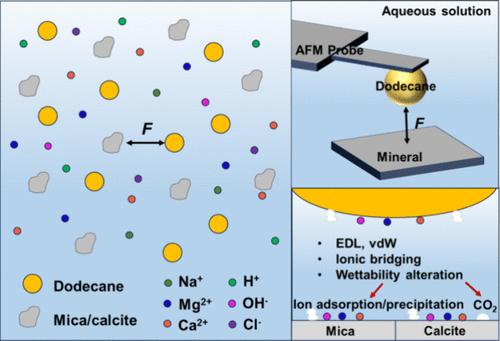
探索十二烷与云母/方解石的相互作用机制:对石油采收率的影响
了解石油与矿物的界面相互作用机理对石油生产和环境保护的应用具有重要意义。本文采用基于原子力显微镜的滴探针技术,研究了十二烷与云母和方解石在水介质中的相互作用。对于十二烷与云母的相互作用,在10 mM NaCl溶液中,电双层斥力占主导地位,较高的pH有利于十二烷的分离。随着NaCl浓度的升高,EDL相互作用减弱,范德华引力(vdW)引起云母表面的油附着。在Ca2+和Mg2+存在的情况下,EDL斥力减弱,导致水膜变薄,并发生油附着。对于十二烷-方解石的相互作用,在所有的溶液条件下都观察到较大的粘附,这主要归因于方解石溶解过程中形成的Ca2+离子桥接。在pH为4时,方解石表面出现CO2气泡,导致十二烷与表面产生排斥性的vdW相互作用,阻碍了方解石的粘附。在高浓度NaCl的作用下,方解石的表面润湿性发生了改变,从而降低了方解石与油的附着力。在500 mM的Ca2+和Mg2+溶液中,离子桥接效应增强,黏附力增强。pH值为10时,方解石表面出现Ca(OH)2(s)和Mg(OH)2(s)的沉淀,降低了方解石的附着力。我们的研究结果为油水矿物相互作用的机制提供了基本的见解,有助于理解和调节提高石油污染物去除和石油采收率的工程过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


