Multisite synergistic interaction induced selective adsorption of CB5-Ti3C2T2 complex for strontium ion: a combined theoretical and experimental study
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
In this work, we use a well-defined water-soluble macrocyclic molecule cucurbit[5]uril (CB5) to modify 2D Ti3C2T2 MXene and assemble a novel high-performance adsorbent CB5-Ti3C2T2 for Sr ion by density functional theory and experimental methods. The structural stabilities of two distinct types of CB5-Ti3C2T2 (T = F, O and OH) complexes, i.e., CB5-Ti3C2T2(V) and CB5-Ti3C2T2(P) configurations are proved by binding energy and ab initio molecular dynamics (AIMD) simulations. Calculations of adsorption properties reveal that all the considered CB5-Ti3C2T2 complexes can act as efficient adsorbents for Sr ion, among which CB5-Ti3C2O2 complex possesses the best performance. The high affinity (the calculated adsorption energies < −7.6 eV) and selectivity of CB5-Ti3C2T2 complex for Sr ion are attributed to the synergistic effect between CB5 molecule and Ti3C2T2 MXene in the adsorption process, which arises from the multisite interactions of portal carbonyl groups of CB5 and surface functional groups of Ti3C2T2 towards Sr. Finally, CB5-Ti3C2T2 complex was successfully synthesized, and experimental results confirm its synergistic effect, good selectivity and high removal efficiency for Sr ions. In the treatment of strontium-containing wastewater with low concentrations, the distribution coefficient and decontamination factor of CB5-Ti3C2T2 for Sr were determined to be as high as 2.88×105 mL/g and 144.9, respectively, and the separation factor (SFSr/Ca) achieved a notable value of 67. 5. This work is expected to present a new strategy for the construction of high-performance MXene-based adsorbent for radionuclide elimination.
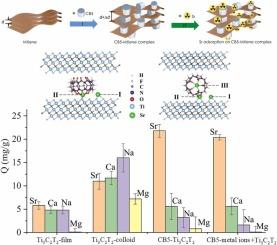
多位点协同作用诱导CB5-Ti3C2T2配合物选择性吸附锶离子:理论与实验相结合的研究
在本研究中,我们利用定义明确的水溶性大环分子cucbit [5]uril (CB5)对2D Ti3C2T2 MXene进行修饰,并通过密度泛函数理论和实验方法组装了一种新型的高性能Sr离子吸附剂CB5-Ti3C2T2。通过结合能和从头算分子动力学(AIMD)模拟,证明了CB5-Ti3C2T2(T = F, O和OH)配合物CB5-Ti3C2T2(V)和CB5-Ti3C2T2(P)两种不同构型的结构稳定性。吸附性能计算表明,所考虑的CB5-Ti3C2T2配合物均能作为Sr离子的高效吸附剂,其中CB5-Ti3C2O2配合物的吸附性能最好。高亲和力(计算吸附能<;−7.6 eV)和CB5-Ti3C2T2配合物对Sr离子的选择性归因于CB5分子与Ti3C2T2 MXene在吸附过程中对Sr离子的协同作用,这种协同作用是由CB5的入口羰基与Ti3C2T2的表面官能团对Sr离子的多位点相互作用产生的。最后,成功合成了CB5-Ti3C2T2配合物,实验结果证实了其协同作用,对Sr离子具有良好的选择性和高的去除效率。在处理低浓度含锶废水时,CB5-Ti3C2T2对Sr的分配系数和去污因子分别高达2.88×105 mL/g和144.9,分离因子(SFSr/Ca)达到了显著值67。5. 这项工作有望为构建高性能的mxeni基放射性核素消除吸附剂提供新的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
文献相关原料
公司名称
产品信息
阿拉丁
LiF
阿拉丁
HCl
阿拉丁
NaCl
阿拉丁
CaCl2
阿拉丁
MgCl2
阿拉丁
SrCl2
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


