Simultaneous and Ultraspecific Optical Detection of Multiple miRNAs Using a Liquid Flow-Based Microfluidic Assay
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Recent studies have reported that the cause and progression of many diseases are closely related to complex and diverse gene regulation involving multiple microRNAs (miRNAs). However, most existing methods for miRNA detection typically deal with one sample at a time, which limits the achievement of high diagnostic accuracy for diseases associated with multiple gene dysregulations. Herein, we develop a liquid flow-based microfluidic optical assay for the simple and reliable detection of two different target miRNAs simultaneously at room temperature without any enzymatic reactions. This assay utilizes the catalytic hairpin assembly cycling reaction in a mixture containing four types of hairpin DNAs to amplify two different dimeric DNA probes, each of which specifically recognizes one of the two different target miRNAs. The resultant two dimeric DNA probes effectively hybridize with anchor DNA grafted into two outlet channels of a microfluidic device, thus enabling i-motif-driven compact DNA hydrogels to form in the channels under acidic conditions. With this setup, the presence of two target miRNAs can be confirmed by the naked-eye observation of red-colored gold nanoparticles encountering a flow blockage in the two outlet channels. Notably, the developed assay demonstrates sensitive and sequence-specific detection that can precisely distinguish a single base mismatch mutant miRNA within 1.5 h. Our assay thus has the potential to serve as a powerful sensing platform for the simple and simultaneous detection of multiple miRNAs in clinical diagnostics at room temperature without analytic equipment or enzymatic reactions.
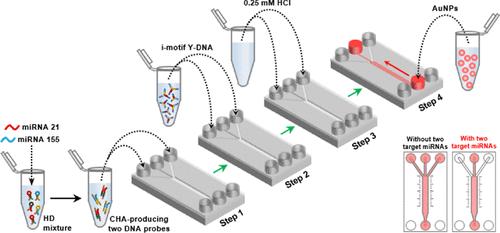
使用基于液体流动的微流体分析同时和超特异的光学检测多种mirna
近年来的研究报道,许多疾病的发生和发展与涉及多个microRNAs (miRNAs)的复杂多样的基因调控密切相关。然而,大多数现有的miRNA检测方法通常一次只处理一个样本,这限制了对与多基因失调相关的疾病的高诊断准确性。在此,我们开发了一种基于液体流动的微流体光学检测方法,可以在室温下同时检测两种不同的靶mirna,而不需要任何酶促反应。该试验利用催化发夹组装循环反应,在含有四种类型的发夹DNA的混合物中扩增两种不同的二聚体DNA探针,每种探针特异性识别两种不同的靶mirna之一。所得到的两个二聚体DNA探针与嫁接到微流控装置的两个出口通道中的锚定DNA有效杂交,从而使i-motif驱动的致密DNA水凝胶在酸性条件下在通道中形成。通过这种设置,可以通过肉眼观察在两个出口通道中遇到流动阻塞的红色金纳米颗粒来确认两个目标mirna的存在。值得注意的是,开发的检测方法具有敏感性和序列特异性,可以在1.5小时内精确区分单个碱基错配突变miRNA。因此,我们的检测方法有潜力作为一个强大的传感平台,在室温下的临床诊断中,无需分析设备或酶促反应即可简单同时检测多个miRNA。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


