Exploring the Structure–Function Relationship in Iridium–Cobalt Oxide Catalyst for Oxygen Evolution Reaction across Different Electrolyte Media
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
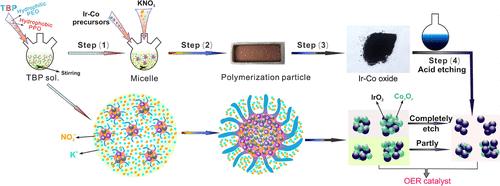
Renewable hydrogen generation from water electrolysis offers a viable path to decarbonization if the costs can be reduced. The iridium-based anode catalyst is one of the most expensive components in electrolyzers. We propose reducing iridium usage by substituting Ir with Co, a more affordable metal, in the mixed oxide phase to enhance the catalytic activity while minimizing Ir consumption. A modified surfactant-assisted Adams fusion synthesis technique was developed as a scalable method for producing IrCo oxide nanoparticles. The synthesized material outperforms the commercial baseline, iridium oxide with carbon (IrOx_C), in both acidic and alkaline media. Acid etching (IrCo_ae) further enhances activity by selectively removing Co to expose more active sites. IrCo_ae achieved a significantly lower overpotential at 10 mA/cm2 compared to IrOx_C, with reductions of approximately 18% under acidic conditions and 14% under alkaline conditions. This work demonstrates that the proposed synthesis method enables efficient Ir utilization and can be adapted to enhance catalyst stability for renewable hydrogen production.
不同电解质介质析氧反应中铱-钴氧化物催化剂的结构-功能关系研究
如果成本可以降低,可再生水电解制氢为脱碳提供了一条可行的途径。铱基阳极催化剂是电解槽中最昂贵的部件之一。我们建议通过在混合氧化物相中用Co(一种更便宜的金属)取代Ir来减少铱的使用,以提高催化活性,同时最大限度地减少Ir的消耗。提出了一种表面活性剂辅助Adams融合合成技术,作为一种可扩展的合成氧化钛纳米颗粒的方法。合成的材料在酸性和碱性介质中都优于商业基准,含碳氧化铱(IrOx_C)。酸蚀(IrCo_ae)通过选择性去除Co以暴露更多活性位点进一步提高活性。与IrOx_C相比,IrCo_ae在10 mA/cm2时的过电位明显降低,在酸性条件下降低了约18%,在碱性条件下降低了14%。这项工作表明,所提出的合成方法能够有效地利用Ir,并可用于提高可再生制氢催化剂的稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


