Highly efficient activation of peroxides by Fe-BDC-NH2 encapsulated nano zero-valent iron composite catalyst for the degradation of chlorophenols
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Chlorinated pollutants, particularly chlorophenols, present a significant concern to ecosystems due to their persistence and toxicity, necessitating the development of effective methods for their elimination from water bodies. In this study, we synthesized a novel nZVI@Fe-MIL-101 catalyst through the reduction of nZVI by NaBH4 and its subsequent encapsulation within Fe-MIL-101, creating a unique core–shell structure. The synthesized nZVI@Fe-MIL-101 catalyst could be effectively utilized for the removal of 2,4-dichlorophenol via an activated hydrogen peroxide-mediated Fenton reaction. The synthesized nZVI@Fe-MIL-101 demonstrated a remarkable enhancement in catalytic performance, with an 11.6-fold increase in the chlorophenol removal rate compared to the unmodified MIL-101(Fe) catalyst. Comprehensive mechanistic investigations using XPS and EPR revealed that nZVI served as the main active substance, with the Fe(II)/Fe(III) cycle providing the primary driving force for H2O2 activation. The π-π interactions between the catalyst and 2,4-dichlorophenol molecules facilitated effective adsorption of 2,4-dichlorophenol onto the catalyst surface. Moreover, the Fe-BDC-NH2 skeleton not only serves a protective function but also, through the incorporation of nitrogen via the amino group, offered a reactive site for the generation of 1O2 and facilitated electron transfer, further activating O2 to produce 1O2. the 2,4-dichlorophenol molecule was ultimately degraded by the combined action of ⋅OH radicals and 1O2 non-radicals. This study demonstrates that nZVI@Fe-MIL-101 exhibits excellent stability and recyclability, making it a promising multiphase catalyst for the treating of chlorophenolic compounds.
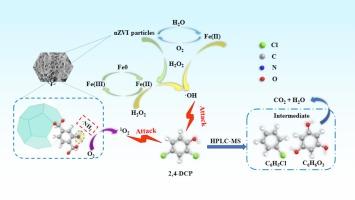
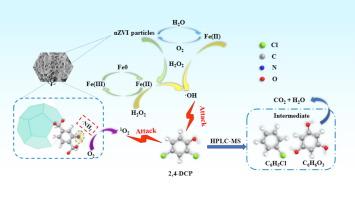
Fe-BDC-NH2包封纳米零价铁复合催化剂对过氧化物的高效活化降解氯酚
氯化污染物,特别是氯酚,由于其持久性和毒性,对生态系统造成重大关切,因此必须发展有效的方法将其从水体中消除。在本研究中,我们通过NaBH4还原nZVI并将其封装在Fe-MIL-101中,合成了一种新型nZVI@Fe-MIL-101催化剂,形成了独特的核壳结构。合成的nZVI@Fe-MIL-101催化剂可以通过活化过氧化氢介导的Fenton反应有效地去除2,4-二氯苯酚。合成的nZVI@Fe-MIL-101催化剂对氯酚的去除率比未改性的MIL-101(Fe)催化剂提高了11.6倍。利用XPS和EPR进行的综合机理研究表明,nZVI是主要的活性物质,Fe(II)/Fe(III)循环是H2O2活化的主要驱动力。催化剂与2,4-二氯苯酚分子之间的π-π相互作用促进了2,4-二氯苯酚在催化剂表面的有效吸附。此外,Fe-BDC-NH2骨架不仅具有保护功能,还通过氨基结合氮,为生成1O2提供反应位点,促进电子转移,进一步激活O2生成1O2。2,4-二氯酚分子最终在⋅OH自由基和1O2非自由基的共同作用下被降解。该研究表明nZVI@Fe-MIL-101具有良好的稳定性和可回收性,是一种很有前途的处理氯酚类化合物的多相催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


