Propagation of neuronal micronuclei regulates microglial characteristics
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
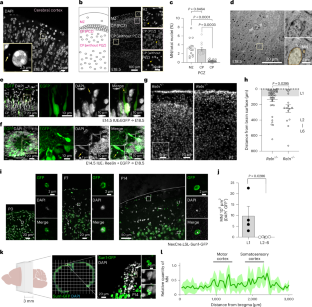
Microglia—resident immune cells in the central nervous system—undergo morphological and functional changes in response to signals from the local environment and mature into various homeostatic states. However, niche signals underlying microglial differentiation and maturation remain unknown. Here, we show that neuronal micronuclei (MN) transfer to microglia, which is followed by changing microglial characteristics during the postnatal period. Neurons passing through a dense region of the developing neocortex give rise to MN and release them into the extracellular space, before being incorporated into microglia and inducing morphological changes. Two-photon imaging analyses have revealed that microglia incorporating MN tend to slowly retract their processes. Loss of the cGAS gene alleviates effects on micronucleus-dependent morphological changes. Neuronal MN-harboring microglia also exhibit unique transcriptome signatures. These results demonstrate that neuronal MN serve as niche signals that transform microglia, and provide a potential mechanism for regulation of microglial characteristics in the early postnatal neocortex. Neuronal micronuclei are transferred to microglia during the early postnatal period, which leads to altered microglial morphology and transcriptomic signatures, suggesting that these micronuclei may act as mediators that control microglial characteristics.

神经元微核的增殖调节着小胶质细胞的特性
中枢神经系统中的小胶质驻留免疫细胞响应局部环境的信号,发生形态和功能变化,并成熟到各种稳态状态。然而,小胶质细胞分化和成熟背后的生态位信号仍然未知。在这里,我们发现神经元微核(MN)转移到小胶质细胞,随后在出生后改变小胶质细胞的特征。神经元通过发育中的新皮质的密集区域产生MN并将其释放到细胞外空间,然后被纳入小胶质细胞并诱导形态变化。双光子成像分析显示,含有MN的小胶质细胞倾向于缓慢地缩回其过程。cGAS基因的缺失减轻了对微核依赖性形态变化的影响。神经元mn窝藏小胶质细胞也表现出独特的转录组特征。这些结果表明,神经元MN作为小胶质细胞转化的利基信号,为出生后早期新皮层小胶质细胞特征的调控提供了潜在的机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


