Cascade Aza-Prins/Friedel–Crafts Reaction of Homocinnamyloxycarbamate and Aromatic Aldehydes Yielding Aromatic Ring-Annulated Hydrocyclopenta-1,2-oxazinane
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The cascade aza-Prins/Friedel–Crafts reaction of homocinnamyloxycarbamate with electron-rich aromatic aldehydes has been successfully established. Most of the aromatic aldehydes react with the carbamate stereoselectively to generate cis-hydroindeno-1,2-oxazinanes. However, the cascade reactions of benzaldehydes bearing two methoxy groups at the meta-positions exhibit a unique stereochemical profile. Furthermore, the cascade reaction of benzaldehyde bearing three methoxy groups at the meta- and para-positions proceeds along with a skeletal rearrangement. Additionally, the aza-Prins/Ritter reaction of cinnamyloxycarbamate with various aldehydes was found during the development of the cascade reaction. In contrast to the cascade aza-Prins/Friedel–Crafts reaction, the aza-Prins/Ritter reaction gave trans-disubstituted isoxazolidines. These stereochemical profiles for both reactions were considered on the basis of several transition-state models.
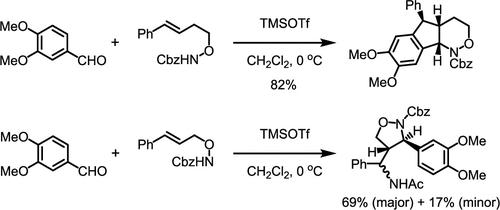
高辛香酰氨基甲酸酯与芳香醛的级联Aza-Prins/ Friedel-Crafts反应生成芳香环环氢环戊-1,2-恶嗪烷
成功地建立了高桂皮酰氨基甲酸酯与富电子芳香醛的aza-Prins/ Friedel-Crafts级联反应。大多数芳香族醛与氨基甲酸酯发生立体选择性反应生成顺式氢茚-1,2-恶嗪烷。然而,间位上含有两个甲氧基的苯甲醛级联反应表现出独特的立体化学特征。此外,在间位和对位上带有三个甲氧基的苯甲醛的级联反应与骨架重排一起进行。此外,在级联反应的发展过程中发现了肉桂酰氨基甲酸酯与多种醛的aza-Prins/Ritter反应。与级联aza-Prins/ Friedel-Crafts反应相比,aza-Prins/Ritter反应得到反式二取代异恶唑烷。这两种反应的立体化学分布是在几个过渡态模型的基础上考虑的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


