Synergizing Process Conditions, Water Sensitivity, and Kinetic Mechanisms to Optimize Sodium Salicylate Yield in Sodium Phenol Carboxylation
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Sodium salicylate can be formed by carboxylation of solid sodium phenol particles with carbon dioxide gas under certain conditions. Single-factor experiments were carried out with self-made dried sodium phenol particles in a batch high-pressure reactor. It was determined that the carboxylation reaction of sodium phenol particles was more suitable under the conditions of a reaction temperature of 160 °C, a reaction pressure of 0.55 MPa, a reaction time of about 40 min, and a stirring speed of 50 rpm. Besides that, the water content of the material also had important effects on the yield. Through the establishment of the kinetic model of the carboxylation reaction between solid sodium phenol particles and carbon dioxide gas, the control step of the reaction temperature at 150 and 160 °C was determined as ash layer diffusion, and the kinetic equation was further calculated. The research results can provide the basic technological conditions and kinetic data of the carboxylation reaction of sodium phenol particles and provide a reference for the development of a continuous and efficient production process of sodium phenol carboxylation.
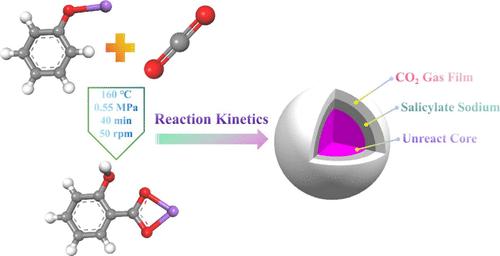
苯酚钠羧化水杨酸钠收率优化的协同工艺条件、水敏感性及动力学机制
在一定条件下,固体苯酚钠颗粒与二氧化碳气体发生羧化反应可生成水杨酸钠。在间歇式高压反应器中,用自制的干燥苯酚钠颗粒进行了单因素实验。结果表明,在反应温度为 160 ℃、反应压力为 0.55 MPa、反应时间约为 40 分钟、搅拌速度为 50 rpm 的条件下,苯酚钠颗粒的羧化反应更为合适。此外,材料的含水量对产率也有重要影响。通过建立固体苯酚钠颗粒与二氧化碳气体发生羧化反应的动力学模型,确定了反应温度在 150 和 160 °C 时的控制步骤为灰层扩散,并进一步计算了动力学方程。该研究成果可提供苯酚钠颗粒羧化反应的基本工艺条件和动力学数据,为开发连续高效的苯酚钠羧化生产工艺提供参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


