Annulative Coupling of Sulfoxonium Ylides with Aldehydes and Naphthols or Coumarins: Easy Access to Fused Dihydrofurans
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We present a novel, metal- and additive-free method for the robust synthesis of dihydrofuran-fused naphthalenes and coumarins. This approach utilizes the annulative coupling of sulfoxonium ylides with aldehydes, naphthols, or coumarins at ambient temperature. The method exhibits broad substrate compatibility, accommodating various functional groups on sulfoxonium ylides and naphthol or coumarin derivatives and resulting in good to high yields of the desired products. Additionally, we successfully scaled up the reactions, and the synthesized derivatives were further converted to other valuable bioactive molecules, validating the viability of our approach.
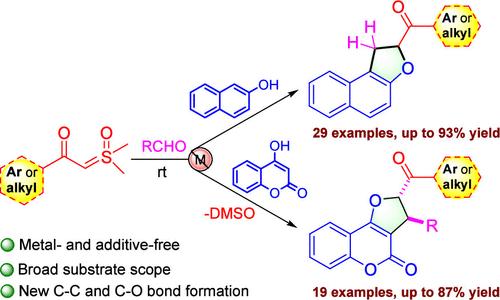
酰基亚砜与醛、萘酚或香豆素的环结偶联:容易得到融合二氢呋喃
我们提出了一种新颖的、不含金属和添加剂的方法,用于强效合成二氢呋喃融合萘和香豆素。这种方法利用了环氧磺酰基与醛、萘酚或香豆素在常温下的环状偶联。该方法具有广泛的底物兼容性,可以在锍酰化物和萘酚或香豆素衍生物上实现各种官能团的偶联,并能获得高产率的所需产物。此外,我们还成功地扩大了反应规模,合成的衍生物被进一步转化为其他有价值的生物活性分子,从而验证了我们方法的可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


