Phase Transformation of Calcium Sulfate at Mineral-Solution Interface: An Overlooked Pathway for Selective Enrichment of Cadmium
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
The reactions at the mineral-solution interface govern whether heavy metals (HMs) ions are retained within minerals or migrate with the solution, thus influencing their cycling and fate. However, the mechanisms driving this differential behavior of HMs at the interface remain poorly understood. In this study, we present a novel paradigm for the selective retention of HMs ions at the mineral-solution interface. By confining the solution on the mineral surface to a defined volume, specifically thinning it down to a thickness of 50 nm, selective retention of Cd ions in the presence of coexisting Cu and Zn ions was achieved. The distribution coefficient of Cd in the mineral reaches as high as 41.44, significantly exceeding that of Cu at 0.13 and Zn at 0.07. Combined with DFT calculations, the results reveal that this selectivity arises from the regulation of the ion desolvation free energy by the solution nanofilm, precisely compensating the energy cost for Cd incorporation as an impurity into the mineral lattice. This work not only enriches the understanding of ion separation behavior at natural mineral-solution interfaces but also offers a new strategy for heavy metal separation and enrichment in industrial applications.
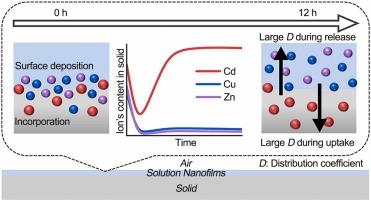
硫酸钙在矿物-溶液界面的相变:一个被忽视的选择性富集镉的途径
矿物-溶液界面的反应决定了重金属(HMs)离子是保留在矿物内部还是随溶液迁移,从而影响其循环和归宿。然而,人们对 HMs 在界面上的这种不同行为的驱动机制仍然知之甚少。在本研究中,我们提出了一种在矿物-溶液界面选择性保留 HMs 离子的新模式。通过将矿物表面的溶液限制在一个确定的体积内,特别是将其厚度减薄至 50 纳米,在铜和锌离子共存的情况下实现了镉离子的选择性保留。镉在矿物中的分布系数高达 41.44,大大超过铜离子的 0.13 和锌离子的 0.07。结合 DFT 计算,结果表明这种选择性源于溶液纳米薄膜对离子解溶解自由能的调节,精确补偿了镉作为杂质加入矿物晶格的能量成本。这项工作不仅丰富了人们对天然矿物-溶液界面离子分离行为的理解,还为工业应用中的重金属分离和富集提供了一种新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


