Versatile platforms of mussel-inspired agarose scaffold for cell cultured meat
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
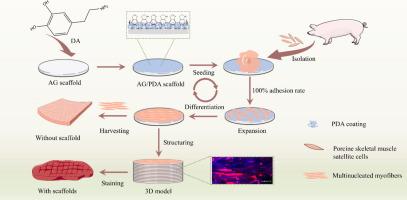
Biomaterial scaffolds are critical for cell cultured meat production. polysaccharide scaffolds lack essential animal cell adhesion receptors, leading to significant challenges in cell proliferation and myogenic differentiation. Thus, enhancing cell adhesion and growth on polysaccharide scaffolds is strongly required to supply the gaps in cell-cultured meat production.Objectives
This study aims to develop a multifunctional cell-responsive hydrogel scaffold for the in vitro production of myofibers and structured cell cultured meat through a “cell adhesion-proliferation-differentiation” strategy.Methods
A polydopamine coating was applied to agarose hydrogel scaffolds using a dipping technique. The capability of scaffolds for myofiber preparation was assessed by evaluating cell adhesion, proliferation, and myogenic differentiation. Utilizing isolated porcine skeletal muscle satellite cells (PSMSCs), the feasibility of structured cell cultured pork tissue supported by agarose hydrogel film scaffolds was further investigated through three-dimensional imaging and scanning electron microscopy analysis. The physicochemical properties of the structured cell cultured pork tissue were evaluated through staining and texture analysis.Results
The incorporation of a polydopamine coating facilitated a remarkable 100 % cell adhesion rate on agarose hydrogel scaffolds, which also demonstrated reusability. The agarose hydrogel scaffolds retained adequate mechanical properties, enabling the adhered cells to proliferate effectively and differentiate into myofiber. Moreover, isolated PSMSCs maintained growth potential on the agarose hydrogel scaffolds, thereby imparting the scaffolds with the ability to generate substantial quantities of multinucleated myofibers. Furthermore, we established a structured cell culture pork meat model, characterized by high-density myofibers and agarose hydrogel film scaffolds, which exhibited the texture and color typical of real pork.Conclusion
The innovative agarose/polydopamine scaffold functions as a multifunctional platform for cell culture, offering novel avenues for the diversification and scalable production of cultured meat, and promising significant reductions in production costs for cell cultured meat.
贻贝启发琼脂糖支架细胞培养肉的多功能平台
多糖支架缺乏必要的动物细胞粘附受体,导致细胞增殖和成肌分化面临重大挑战。本研究旨在通过 "细胞粘附-增殖-分化 "策略,开发一种多功能细胞响应水凝胶支架,用于体外生产肌纤维和结构化细胞培养肉。通过评估细胞粘附、增殖和成肌分化情况,评估了支架制备肌纤维的能力。利用分离的猪骨骼肌卫星细胞(PSMSCs),通过三维成像和扫描电子显微镜分析,进一步研究了由琼脂糖水凝胶膜支架支撑的猪肉组织结构化细胞培养的可行性。结果聚多巴胺涂层的加入使琼脂糖水凝胶支架上的细胞粘附率达到了显著的 100%,这也证明了其可重复使用性。琼脂糖水凝胶支架保持了足够的机械性能,使粘附的细胞能有效增殖并分化成肌纤维。此外,分离出的 PSMSCs 还能在琼脂糖水凝胶支架上保持生长潜能,从而赋予支架生成大量多核肌纤维的能力。结论 创新的琼脂糖/多巴胺支架可作为细胞培养的多功能平台,为培养肉的多样化和规模化生产提供了新途径,并有望显著降低细胞培养肉的生产成本。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
文献相关原料
公司名称
产品信息
索莱宝
horse serum
索莱宝
alamar blue cell viability assay reagent
索莱宝
Triton-100
索莱宝
collagenase type I digestion solution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


