Synthesis of single-crystal UiO-67-(NH2)2 for effective SO2 adsorption and separation from flue gas
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The synthesis of stable and efficient porous adsorbents for adsorptive capture and recovery of SO2 from flue gas is of great importance for minimizing air pollution and reducing costs. Herein, by choosing trifluoroacetic acid as modulator, the single-crystal UiO-67-(NH2)2 has been successfully synthesized benefitting crystal structure determination. The stability tests, gas adsorption measurements, and breakthrough experiments demonstrated that single-crystal UiO-67-(NH2)2 possesses moderate acid-base and hydrothermal stability, and exhibited high SO2 uptake (19.72 mmol/g, 298 K and 1 bar), good SO2/CO2 separation selectivity (110.3~33.3, SO2/CO2 = 10/90), and excellent SO2 recovery purity (93.3 %). However, the instability of the UiO-67-(NH2)2 framework, combined with the strong corrosive and reactive properties of SO2, can lead to partial collapse of the framework during the adsorption and capture process. This ultimately results in a decreased SO2 capture performance over time. In-situ DRIFT, GCMC simulation and DFT calculations revealed that the μ3-OH of Zr6O4(OH)4 clusters and the —NH2 groups of the ligand can form multiple hydrogen bonds with SO2 molecules. This ensures that SO2 molecules are firmly grasped by UiO-67-(NH2)2 framework and facilitate the efficiently selective capture of SO2 at low concentration.
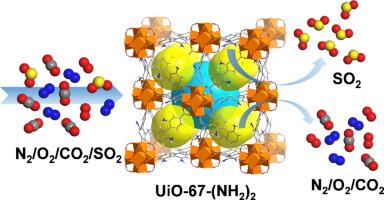
单晶UiO-67-(NH2)2的合成及其对烟气中二氧化硫的有效吸附和分离
合成稳定高效的多孔吸附剂,用于吸附捕集和回收烟气中的二氧化硫,对于减少空气污染和降低成本具有重要意义。本文以三氟乙酸为调制剂,成功合成了单晶 UiO-67-(NH2)2,并进行了晶体结构测定。稳定性测试、气体吸附测量和突破实验表明,单晶 UiO-67-(NH2)2 具有中等的酸碱稳定性和水热稳定性,并表现出较高的 SO2 吸收率(19.72 mmol/g,298 K 和 1 bar)、良好的 SO2/CO2 分离选择性(110.3~33.3,SO2/CO2 = 10/90)和优异的 SO2 回收纯度(93.3 %)。然而,UiO-67-(NH2)2 框架的不稳定性,加上 SO2 的强腐蚀性和反应特性,会导致框架在吸附和捕获过程中部分坍塌。随着时间的推移,这最终会导致二氧化硫捕获性能下降。原位 DRIFT、GCMC 模拟和 DFT 计算表明,Zr6O4(OH)4 团簇的μ3-OH 和配体的 -NH2 基团可与 SO2 分子形成多个氢键。这确保了二氧化硫分子被 UiO-67-(NH2)2 框架牢牢抓住,并促进了低浓度二氧化硫的高效选择性捕获。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


