IL-33-activated ILC2s induce tertiary lymphoid structures in pancreatic cancer
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
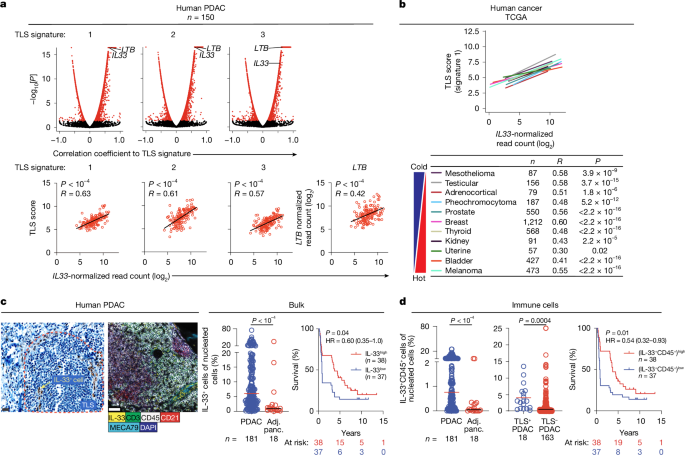
Tertiary lymphoid structures (TLSs) are de novo ectopic lymphoid aggregates that regulate immunity in chronically inflamed tissues, including tumours. Although TLSs form due to inflammation-triggered activation of the lymphotoxin (LT)–LTβ receptor (LTβR) pathway1, the inflammatory signals and cells that induce TLSs remain incompletely identified. Here we show that interleukin-33 (IL-33), the alarmin released by inflamed tissues2, induces TLSs. In mice, Il33 deficiency severely attenuates inflammation- and LTβR-activation-induced TLSs in models of colitis and pancreatic ductal adenocarcinoma (PDAC). In PDAC, the alarmin domain of IL-33 activates group 2 innate lymphoid cells (ILC2s) expressing LT that engage putative LTβR+ myeloid organizer cells to initiate tertiary lymphoneogenesis. Notably, lymphoneogenic ILC2s migrate to PDACs from the gut, can be mobilized to PDACs in different tissues and are modulated by gut microbiota. Furthermore, we detect putative lymphoneogenic ILC2s and IL-33-expressing cells within TLSs in human PDAC that correlate with improved prognosis. To harness this lymphoneogenic pathway for immunotherapy, we engineer a recombinant human IL-33 protein that expands intratumoural lymphoneogenic ILC2s and TLSs and demonstrates enhanced anti-tumour activity in PDAC mice. In summary, we identify the molecules and cells of a druggable pathway that induces inflammation-triggered TLSs. More broadly, we reveal a lymphoneogenic function for alarmins and ILC2s. IL-33 induces tertiary lymphoid structures.

il -33激活的ILC2s在胰腺癌中诱导三级淋巴结构
三级淋巴样结构(TLSs)是一种新的异位淋巴样聚集体,可调节慢性炎症组织(包括肿瘤)的免疫。虽然TLSs的形成是由于炎症引发的淋巴感光素(LT) -LTβ受体(LTβR)途径的激活1,但诱导TLSs的炎症信号和细胞仍未完全确定。本研究表明,炎症组织释放的警报素-白细胞介素-33 (IL-33)可诱导TLSs。在小鼠中,Il33缺乏严重减弱结肠炎和胰腺导管腺癌(PDAC)模型中炎症和ltβ r激活诱导的TLSs。在PDAC中,IL-33的报警蛋白结构域激活表达LT的2组先天淋巴样细胞(ILC2s),该细胞与假定的LTβR+髓样组织细胞结合,启动三级淋巴生成。值得注意的是,淋巴源性ILC2s从肠道迁移到pdac,可以被动员到不同组织的pdac,并受到肠道微生物群的调节。此外,我们在人类PDAC的TLSs中检测到推定的淋巴源性ILC2s和il -33表达细胞,这些细胞与预后改善有关。为了利用这种淋巴原性途径进行免疫治疗,我们设计了一种重组人IL-33蛋白,该蛋白可以扩展肿瘤内淋巴原性ILC2s和TLSs,并在PDAC小鼠中显示出增强的抗肿瘤活性。总之,我们确定了可诱导炎症触发TLSs的药物通路的分子和细胞。更广泛地说,我们揭示了警报器和ILC2s的淋巴生成功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


