Vibrational weak and strong coupling modify a chemical reaction via cavity-mediated radiative energy transfer
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
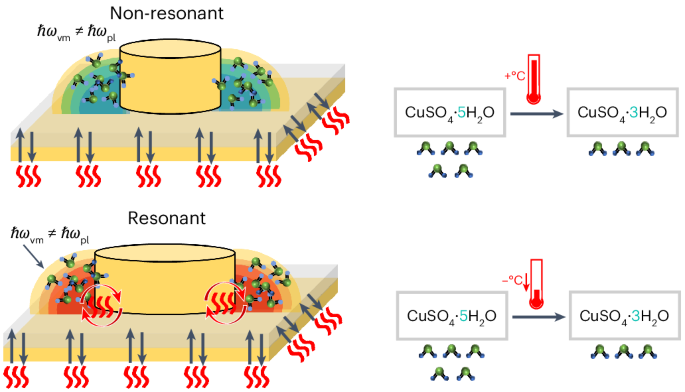
Controlling reaction outcomes through external influences is a central goal in chemistry. Vibrational coupling between molecular vibrations and cavity modes is rapidly emerging as a distinct strategy compared with conventional thermochemical and photochemical methods; however, insight into the fundamental mechanisms remains limited. Here we investigate how vibrational weak and strong coupling in plasmonic nanocavities modifies the thermal dehydration of copper sulfate pentahydrate. We demonstrate that light–matter coupling reduces the onset temperature for dehydration by up to 14 °C, and we attribute this effect to enhanced radiative energy transport that is mediated by resonant electromagnetic modes, eliminating temperature gradients in the coupled system. Our findings provide direct evidence of localized energy transfer leading to modified chemical behaviour in specific regions of high optical density of states. This work establishes a mechanism for modifying thermally driven chemical processes using optical cavities, with implications for the development of catalytic systems that exploit these tailored interactions to achieve targeted reaction control. Vibrational weak and strong light–matter coupling in infrared nanocavities modifies chemical processes. Now it has been shown that this coupling can control thermally driven reactions through enhanced radiative energy transport.

振动弱耦合和强耦合通过腔体介导的辐射能量传递改变化学反应
通过外部影响控制反应结果是化学的中心目标。与传统的热化学和光化学方法相比,分子振动和腔模式之间的振动耦合正迅速成为一种独特的策略;然而,对基本机制的了解仍然有限。在这里,我们研究了等离子体纳米腔中的振动弱耦合和强耦合如何改变五水硫酸铜的热脱水。我们证明,光-物质耦合降低了脱水的起始温度高达14°C,我们将这种效应归因于共振电磁模式介导的辐射能量传输增强,消除了耦合系统中的温度梯度。我们的发现提供了局部能量转移导致高光密度态特定区域化学行为改变的直接证据。这项工作建立了一种利用光学腔来修改热驱动化学过程的机制,这对开发利用这些定制相互作用来实现目标反应控制的催化系统具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


