Cationic peptides cause memory loss through endophilin-mediated endocytosis
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
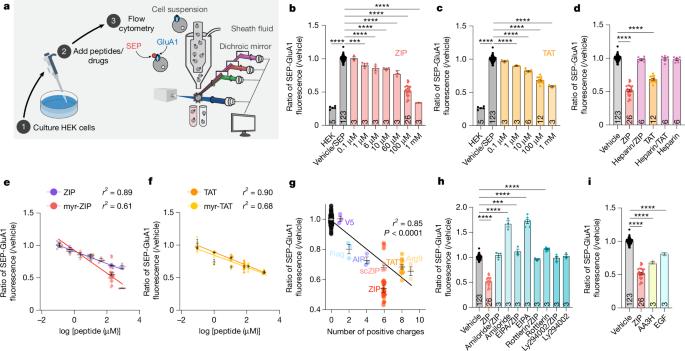
The zeta inhibitory peptide (ZIP) interferes with memory maintenance and long-term potentiation (LTP)1 when administered to mice. However, mice lacking its putative target, protein kinase PKMζ, exhibit normal learning and memory as well as LTP2,3, making the mechanism of ZIP unclear. Here we show that ZIP disrupts LTP by removing surface AMPA receptors through its cationic charge alone. This effect requires endophilin-A2-mediated endocytosis and is fully blocked by drugs suppressing macropinocytosis. ZIP and other cationic peptides remove newly inserted AMPA receptor nanoclusters at potentiated synapses, providing a mechanism by which these peptides erase memories without altering basal synaptic function. When delivered in vivo, cationic peptides can modulate memories on local and brain-wide scales, and these mechanisms can be leveraged to prevent memory loss in a model of traumatic brain injury. Our findings uncover a previously unknown synaptic mechanism by which memories are maintained or lost. Elucidation of the mechanism by which zeta inhibitory peptide erases memories, involving endocytosis of AMPA receptors on potentiated synapses, provides insight into more general mechanisms of memory maintenance and response to traumatic brain injury.
阳离子多肽通过内啡肽介导的内吞作用引起记忆丧失
zeta抑制肽(ZIP)干扰小鼠的记忆维持和长期增强(LTP)1。然而,缺乏其假定靶点PKMζ蛋白激酶的小鼠表现出正常的学习和记忆以及LTP2,3,这使得ZIP的机制尚不清楚。在这里,我们表明ZIP通过其阳离子电荷单独去除表面AMPA受体来破坏LTP。这种作用需要内啡肽- a2介导的内吞作用,并被抑制巨噬细胞增多的药物完全阻断。ZIP和其他阳离子肽在增强突触中去除新插入的AMPA受体纳米簇,提供了一种机制,通过这种机制,这些肽在不改变基础突触功能的情况下消除记忆。当在体内传递时,阳离子肽可以在局部和全脑范围内调节记忆,这些机制可以用来预防创伤性脑损伤模型中的记忆丧失。我们的发现揭示了一种以前未知的突触机制,记忆是通过这种机制维持或丢失的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


