Selective Th(IV) separation and immobilization by one-dimensional lepidocrocite titanate
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
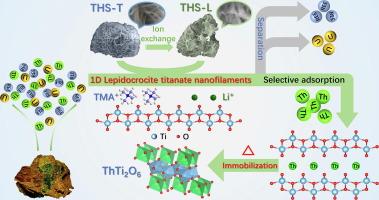
Titanate nanomaterials have been widely explored for the effective removal of heavy metals and radionuclide ions from environmental wastewater, leveraging their high specific surface areas (SSAs) and ion exchange capacities. In this study, one-dimensional lepidocrocite titanate hierarchical structures (THSs) incorporating exchangeable Li+ or tetramethylammonium (TMA+), cations were prepared using an organic alkali conversion strategy suitable for large-scale production. These unique adsorbents were tested for the efficient capture and separation of Th(IV). Batch adsorption experiments revealed that the maximum adsorption capacity of THSs for Th(IV) reached 292 mg/g at pH 2.5. THSs demonstrated superior ion selectivity for Th(IV) over uranyl ions and rare earth ions, achieving a Th(IV)/U(VI) separation factor of up to 722. In the context of treating simulated rare earth ore leachates, the corresponding Th(IV) adsorption rate and Kd values were recorded at 98.4 % and 1.52 × 105 mL/g, respectively. Spectroscopic analyses, including XPS and EXAFS, complemented by DFT calculations, substantiated that Th(IV) interacts stably with the oxygen-containing terminations on the surface of THSs, primarily through the formation of inner-sphere complexations. Sintering of Th-loaded THSs led to the formation of stable ThTi2O6 phases, facilitating the complete immobilization of Th(IV) within the lattice, with a leaching rate of less than 0.05 % under highly acidic conditions. Overall, THSs demonstrate significant potential for the efficient purification of Th-containing wastewater from rare earth mines, as well as for the secure geological disposal of the resultant waste.
钛酸鳞片石的选择性分离与固定化
钛酸盐纳米材料利用其高比表面积(SSAs)和离子交换能力,在有效去除环境废水中的重金属和放射性核素离子方面得到了广泛的研究。在本研究中,采用适合大规模生产的有机碱转化策略,制备了含有可交换Li+或四甲基铵(TMA+)阳离子的一维钛酸鳞石分层结构(THSs)。这些独特的吸附剂对Th(IV)的有效捕获和分离进行了测试。批量吸附实验表明,在pH为2.5时,THSs对Th(IV)的最大吸附量为292 mg/g。THSs对Th(IV)的选择性优于铀酰离子和稀土离子,Th(IV)/U(VI)的分离系数高达722。在处理模拟稀土矿渗滤液的条件下,相应的Th(IV)吸附率和Kd值分别为98.4% %和1.52 × 105 mL/g。光谱分析,包括XPS和EXAFS,辅以DFT计算,证实了Th(IV)与THSs表面的含氧末端稳定地相互作用,主要是通过形成内球配合物。负载Th的THSs烧结形成稳定的ThTi2O6相,有利于Th(IV)在晶格内的完全固定,在强酸性条件下浸出率小于0.05 %。总的来说,THSs在有效净化稀土矿的含钍废水以及对所产生的废物进行安全的地质处置方面显示出巨大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


