Concentration-Dependent Control of the Band Gap Energy of a Low-Dimensional Lepidocrocite Titanate
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
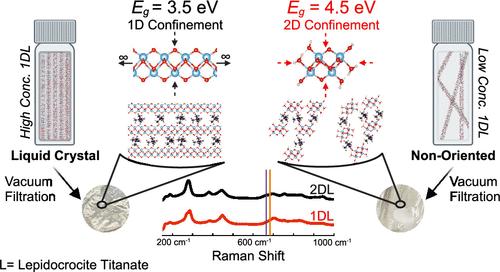
Recently, we reported on the simple, scalable synthesis of quantum-confined one-dimensional (1D) lepidocrocite titanate nanofilaments (1DLs). Herein, we show, using solid-state UV–vis spectroscopy, that reducing the concentration of aqueous 1DL colloidal suspensions from 40 to 0.01 g/L increases the band gap energy and light absorption onset of dried filtered films from ≈3.5 to ≈4.5 eV. This range is ascribed to quantum confinement as the system transitions from two-dimensional (2D) into 1D with dilution. It is only after the colloidal suspensions are dried and the 1DLs start to self-assemble into ribbons and sheets that the band gap values change. This self-assembly is manifested in the X-ray diffraction patterns and the emergence of a Raman band characteristic of 2D lepidocrocite titanates. In colloidal form, 1DLs exhibit a lyotropic liquid crystal phase with a critical concentration of between 10 and 1 g/L. Additionally, the Beer–Lambert law applies with a mass absorbance coefficient of 2 ± 0.4 Lg–1 cm–1. The optical absorbance edges of the colloidal suspensions are not a function of concentration. The experimental findings are theoretically supported by density functional theory calculations of the Raman vibrational modes and electronic band structures of the 1D and 2D lepidocrocite titanate atomic structures.
低维钛酸蛭石带隙能量的浓度依赖性控制
最近,我们报道了一种简单的、可扩展的量子约束一维(1D)钛酸鳞片石纳米丝(1dl)的合成方法。本文中,我们使用固态紫外-可见光谱技术表明,将1DL胶体悬浮液的水溶液浓度从40 g/L降低到0.01 g/L,使干燥过滤膜的带隙能量和光吸收起始值从≈3.5 eV增加到≈4.5 eV。这个范围归因于量子约束,因为系统从二维(2D)过渡到一维,并有稀释。只有在胶体悬浮液干燥后,1dl开始自组装成带状和片状后,带隙值才会发生变化。这种自组装表现在二维钛酸蛭石的x射线衍射图和拉曼带特征的出现上。在胶体形态下,1dl表现为溶致液晶相,临界浓度在10 ~ 1g /L之间。此外,比尔-朗伯定律适用于质量吸光度系数为2±0.4 lb - 1 cm-1。胶体悬浮液的光学吸光度边缘不是浓度的函数。实验结果在理论上得到了钛酸蛭石一维和二维原子结构拉曼振动模式和电子能带结构的密度泛函理论计算的支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


