Highly Efficient Bifunctional NiFe-MOF Array Electrode for Nitrate Reduction to Ammonia and Oxygen Evolution Reactions
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Electrocatalytically converting nitrates in sewage to ammonia, which can not only achieve the purpose of eliminating sewage but also obtaining valuable ammonia, is an effective supplement to the traditional Haber–Bosch process. Although significant progress has been made in cathodic catalyst design, the overall ammonia electrolysis from nitrate reduction is still restricted by the anodic oxygen evolution heavily relying on noble-based catalysts. Herein, a bimetallic NiFe-MOF nanosheet array electrode is fabricated and serves as an efficient bifunctional catalyst for nitrate reduction and oxygen evolution reactions. The introduction of Fe to Ni-MOF facilitates the formation of a nanosheet structure with higher electrochemical active surface area, as well as provides synergetic NiFe sites. The NiFe-MOF electrode reaches a greatly enhanced ammonia yield rate of 0.94 mmol cm–2 h–1 and a Faradaic efficiency of 90.8% at the cathode and −0.6 V versus reversible hydrogen electrode, as well as an enhanced oxygen evolution reaction with a declined overpotential of 424 mV at 50 mA cm–2. As a bifunctional catalyst in the overall electrocatalysis, the performance of NiFe-MOF in the nitrate reduction reaction is comparable with that using Pt mesh as a counter electrode.
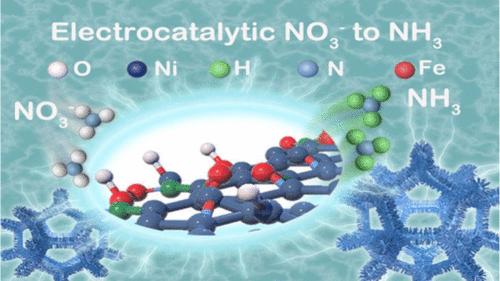
用于硝酸盐还原为氨和析氧反应的高效双功能nfe - mof阵列电极
电催化将污水中的硝酸盐转化为氨,既能达到去除污水的目的,又能获得有价氨,是对传统Haber-Bosch工艺的有效补充。虽然阴极催化剂的设计取得了重大进展,但硝酸盐还原氨的整体电解仍然受到阳极析氧的限制,而阳极析氧严重依赖于noble基催化剂。本文制备了一种双金属nfe - mof纳米片阵列电极,作为硝酸盐还原和析氧反应的高效双功能催化剂。在Ni-MOF中引入Fe有助于形成具有更高电化学活性表面积的纳米片结构,并提供协同的NiFe位点。与可逆氢电极相比,nfe - mof电极在阴极和- 0.6 V下的氨收率为0.94 mmol cm-2 h-1,法拉第效率为90.8%,在50 mA cm-2下过电位下降424 mV,析氧反应增强。作为整体电催化中的双功能催化剂,nfe - mof在硝酸还原反应中的性能与使用Pt网作为对电极的性能相当。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


