Hollow Square Ni-Doped Copper Oxide Catalyst Boosting Electrocatalytic Nitrate Reduction
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The electrochemical nitrate reduction reaction to ammonia (NRA) is gaining increasing attention as an eco-friendly approach to convert harmful nitrate pollutants into high-value product ammonia. NRA involves two critical rate-determining steps: hydrogenation of the *NO and *NOH intermediates. The composite of Ni and Cu has been demonstrated to exhibit synergistic catalytic effects; however, research on the combination of Ni and CuO remains limited. Herein, an advanced Ni-doped copper oxide catalyst with a hollow square morphology (Ni–CuO) is reported with a Faradaic efficiency of 95.26% at −0.8 V vs RHE and a high yield rate of 0.94 mmol h–1 cm–2, demonstrating high selectivity and stability. Complementary analyses demonstrated that the active hydrogen generated at the Ni sites facilitates the hydrogenation of *NOx adsorbed on Cu sites. Theoretical computations further confirm the thermodynamic viability of this bimetallic catalytic mechanism. Furthermore, an Al–NO3– battery with a high open-circuit voltage was constructed by using Ni–CuO as the cathode. This work presents a synergistically modulated catalyst for complex catalytic processes and introduces a highly efficient Al–NO3– battery capable of simultaneous NH3 synthesis and electrical energy conversion, underscoring its potential in efficient catalysis and the development of the energy and chemical industries.
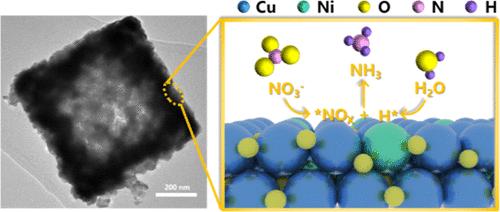
空心方形镍掺杂铜氧化物催化剂促进电催化还原硝酸盐
电化学硝酸还原反应制氨(NRA)作为一种将有害的硝酸盐污染物转化为高价值产品氨的环保方法正受到越来越多的关注。NRA包括两个关键的速率决定步骤:*NO和*NOH中间体的氢化。Ni和Cu的复合材料具有协同催化作用;然而,对Ni和CuO结合的研究仍然有限。在−0.8 V vs RHE条件下,Ni-CuO催化剂的法拉第效率为95.26%,产率为0.94 mmol h-1 cm-2,具有较高的选择性和稳定性。互补分析表明,Ni位点产生的活性氢有利于吸附在Cu位点上的*NOx的加氢。理论计算进一步证实了该双金属催化机理的热力学可行性。以Ni-CuO为阴极,制备了具有高开路电压的Al-NO3 -电池。本研究提出了一种用于复杂催化过程的协同调节催化剂,并介绍了一种能够同时合成NH3和转换电能的高效Al-NO3 -电池,强调了其在高效催化和能源和化学工业发展中的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


