Understanding Substrate Binding and Reactivity of Stearoyl-CoA Desaturase (SCD1) through Classical and Multiscale Molecular Dynamics Simulations
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
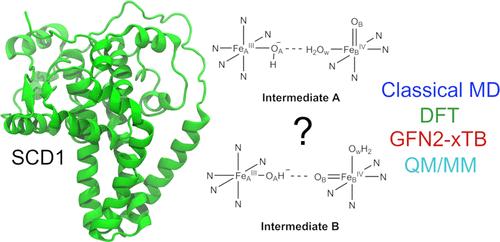
Stearoyl-CoA desaturase (SCD1) plays an important role in the metabolism of fatty acids and is a promising therapeutic target. However, the underlying mechanism of SCD1, as well as other transmembrane nonheme diiron enzymes, remains poorly understood. This study builds upon a previous density functional theory (DFT) cluster model study which proposed a potential reactive intermediate of SCD1. We assessed its dynamical properties by employing classical molecular dynamics (MD) simulations. The simulations revealed that the proposed intermediate lacks the ability to form a favorable reactive complex with stearoyl-CoA, highlighting the significance of a conserved asparagine residue in controlling the substrate’s orientation. Motivated by these observations, we proposed a modified intermediate in which a water molecule is strategically placed to stabilize the conserved asparagine residue. Subsequent classical MD simulations showed that the modified intermediate is able to form a reactive complex with the substrate, consistent with the experimentally observed selectivity of SCD1. A cluster model DFT study showed that the modified intermediate is of similar reactivity as the previously reported intermediate. The free energy barrier for the first hydrogen atom abstraction step by the modified intermediate was estimated to be accessible. The estimate is based on a hybrid quantum mechanics/molecular mechanics (QM/MM) approach utilizing the efficient semiempirical GFN2-xTB method. Considering its computational efficiency, GFN2-xTB seems to be a promising tool for the study of complex transition metal systems. Overall, our findings reveal important structure–function relationships in SCD1, uncovering an interplay between conserved residues and regioselectivity which advances our understanding of the entire class of transmembrane nonheme diiron enzymes.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


