Bacterial Nanovesicles as Interkingdom Signaling Moieties Mediating Pain Hypersensitivity
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
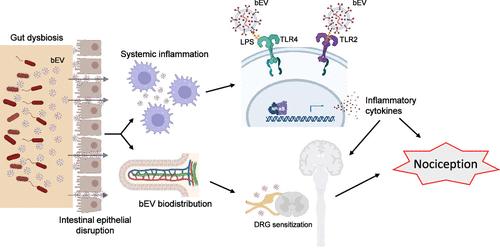
Gut dysbiosis contributes to multiple pathologies, yet the mechanisms of the gut microbiota-mediated influence on systemic and distant responses remain largely elusive. This study aimed to identify the role of nanosized bacterial extracellular vesicles (bEVs) in mediating allodynia, i.e., pain hypersensitivity, in a diet-induced obesity (DIO) gut dysbiosis model. bEVs were enriched from the feces of lean (bEVLean) and DIO (bEVDIO) mice by an approach combining ultracentrifugation and immunoprecipitation and then extensively analyzed for purity and bacterial characteristics. Next, bEVs were injected, either intraplantarly or intravenously, in mice to assess pain sensitivity. Fluorescence-labeled bEVs were injected in mice by enema to assess biodistribution. The effect of bEV on immune cells and inflammation was analyzed by array, immunophenotyping, microscopy, NF-κB activation, and cellular uptake assays. Results showed that bEVDIO administration in wild-type mice replicated the allodynia phenotype observed in DIO mice for both mechanical and thermal stimuli. Importantly, this effect was compromised in TRPA1/TRPV1 double-knockout mice. Biodistribution analyses showed bEV entry into systemic circulation with subsequent localization at distant sites. Multiple analyses revealed that bEVDIO exposure incited systemic inflammation, primarily through modulating the innate immune system. This inflammatory mechanism involved LPS on the bEV surface, activating TLR2- and TLR4-related pathways, as confirmed using TLR2 and TLR4 inhibitors and shaving bEV surface proteins. Interestingly, the enhanced cellular uptake of bEVDIO was contingent on interactions involving LPS and proteins on bEVs and TLR2/TLR4 on monocytes. These findings illuminate the hitherto unexplored role of bEV as pivotal mediators of allodynia and inflammation linked to gut dysbiosis.
细菌纳米囊泡作为界间信号通路介导疼痛超敏反应
肠道生态失调会导致多种病理,但肠道微生物群介导的对全身和远处反应的影响机制在很大程度上仍然难以捉摸。本研究旨在确定纳米细菌细胞外囊泡(bEVs)在饮食性肥胖(DIO)肠道生态失调模型中介导异常性疼痛(即疼痛超敏反应)的作用。采用超离心和免疫沉淀相结合的方法从瘦小鼠(bEVLean)和DIO小鼠(bEVDIO)的粪便中富集bev,并对其纯度和细菌特性进行广泛分析。接下来,将bev以足底或静脉注射的方式注射到小鼠体内,以评估疼痛敏感性。通过灌肠注射荧光标记的bev以评估其生物分布。通过阵列、免疫表型、显微镜、NF-κB活化和细胞摄取分析bEV对免疫细胞和炎症的影响。结果表明,bEVDIO野生型小鼠在机械和热刺激下均复制了DIO小鼠的异常性疼痛表型。重要的是,这种作用在TRPA1/TRPV1双敲除小鼠中被削弱。生物分布分析显示bEV进入体循环,随后在远处定位。多项分析显示,bEVDIO暴露主要通过调节先天免疫系统引发全身性炎症。这种炎症机制涉及到bEV表面的LPS,激活TLR2-和TLR4相关通路,通过使用TLR2和TLR4抑制剂和剃除bEV表面蛋白证实了这一点。有趣的是,bEVDIO的细胞摄取增强取决于bev上的LPS和蛋白质以及单核细胞上的TLR2/TLR4的相互作用。这些发现阐明了迄今为止尚未探索的bEV作为与肠道生态失调相关的异常性疼痛和炎症的关键介质的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


