Efficient Synthesis of l-Asparagine by an Immobilized Three-Enzyme Cascade Reaction System
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
l-Asparagine (l-Asn) is an important amino acid with broad applications in food, medicine, fine chemicals, and environmental protection. However, its industrial production is limited by the high cost of raw materials and the low catalytic efficiency of enzymes. In this study, a three-enzyme cascade pathway (FDN) was designed to produce l-Asn, using fumaric acid as the raw material. Within this pathway, EcAsnA was identified as the rate-limiting enzyme and was subsequently engineered using a product rescue strategy to reduce product inhibition by opening the closed gate. The optimal mutant, L109 K/K58R, exhibited a 6.61-fold reduction in product inhibition and a 4.24-fold improvement in catalytic efficiency. This mutant was then integrated into Escherichia coli along with the other two enzymes to construct the optimal recombinant strain E. coli 17. Using diatomite-glutaraldehyde cross-linking immobilized E. coli 17 as a biocatalyst, 267.74 g of l-Asn (with 5.35 g·L–1·h–1 STY, > 99% ee) was produced across 50 batch feedings in 1 L reaction volume. The purity of l-Asn exceeded 99% after isolation and purification. This study demonstrates the effective integration of cascade reaction design, enzyme engineering, and cell immobilization, providing a promising approach for synthesizing high-value products from cost-effective substrates.
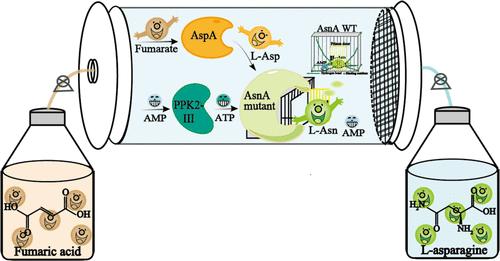
固定化三酶级联反应体系高效合成l-天冬酰胺
l-天冬酰胺(l-Asn)是一种重要的氨基酸,在食品、医药、精细化工和环境保护等领域有着广泛的应用。然而,其工业化生产受到原材料成本高和酶催化效率低的限制。本研究以富马酸为原料,设计了一种三酶级联途径(FDN)来生产 l-氨。在这一途径中,EcAsnA 被确定为限速酶,随后利用产物拯救策略进行了改造,通过打开封闭的闸门来减少产物抑制。最佳突变体 L109 K/K58R 的产物抑制率降低了 6.61 倍,催化效率提高了 4.24 倍。然后将该突变体与其他两种酶整合到大肠杆菌中,构建出最佳重组菌株大肠杆菌 17。使用硅藻土-戊二醛交联固定化大肠杆菌 17 作为生物催化剂,在 1 L 反应体积中,通过 50 次批量进料生产出 267.74 g l-Asn(5.35 g-L-1-h-1 STY,> 99% ee)。经分离和纯化后,l-Asn 的纯度超过 99%。这项研究展示了级联反应设计、酶工程和细胞固定化的有效整合,为利用具有成本效益的底物合成高价值产品提供了一种前景广阔的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


