Clinical Trial: Study to Investigate the Efficacy and Safety of the Alpha-2-Delta Ligand PD-217,014 in Patients With Irritable Bowel Syndrome
Abstract
Introduction
Despite the emergence of drugs to treat irritable bowel syndrome (IBS), improving abdominal pain can still be challenging. α2δ ligands, such as gabapentin and pregabalin, are sometimes used off-label to tackle this problem. However, evidence for efficacy is limited, and no large-scale studies have been published.
Aim
To study the efficacy of the α2δ ligand PD-217,014 in IBS.
Methods
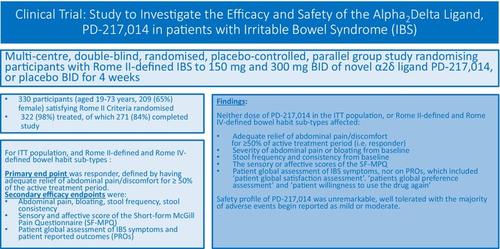
This multi-centre, double-blind, randomised, placebo-controlled, parallel group study randomised participants with Rome II-defined IBS to 150 or 300 mg b.d. of PD-217,014 or placebo b.d. for 4 weeks. The primary efficacy endpoint was responder, defined as having adequate relief of abdominal pain/discomfort for ≥ 50% of the active treatment period. Key secondary endpoints were change from baseline in abdominal pain, bloating, stool frequency/consistency, and global assessment of IBS symptoms.
Results
We randomised 330 participants [aged 19–73 years; 209 (65%) female] satisfying Rome II criteria, 322 (98%) were treated, and of whom 271 (84%) completed the study. In this study, 321 satisfied Rome IV criteria. Neither dose of PD-217,014 improved the percentage of participants reporting adequate relief of abdominal pain/discomfort compared with placebo, either using the Rome II-defined total cohort or Rome II and IV IBS bowel habit sub-types. There were similar observations for secondary endpoints, and no association between abdominal pain or anxiety levels at baseline with participant improvement. PD-217,014 was generally well tolerated.
Conclusion
This first large, dose-ranging trial examining the efficacy of PD-217,014 showed no significant efficacy in participants with IBS or bowel habit sub-types, irrespective of their pain and anxiety levels.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


