Biphasic Coacervation Controlled by Kinetics as Studied by De Novo-Designed Peptides
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
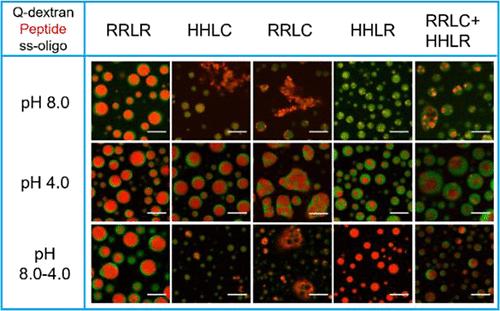
Coacervation is generally treated as a liquid–liquid phase separation process and is controlled mainly by thermodynamics. However, kinetics could make a dominant contribution, especially in systems containing multiple interactions. In this work, using peptides of (XXLY)6SSSGSS to tune the charge density and the degree of hydrophobicity, as well as to introduce secondary structures, we evaluated the effect of kinetics on biphasic coacervates formed by peptides with single-stranded oligonucleotides and quaternized dextran at varying pH values. Only in the case where the charge density is constant and the electrostatic interaction is the major driving force for Coacervation is the effect of kinetics negligible. When pH-dependent electrostatic interaction and hydrophobic interaction are involved or the peptides form secondary structures, the Coacervation process is then path-dependent, indicating that the kinetics controls the phase separation process. The Coacervation by combining two different peptides suggests that the peptide with a higher charge density plays a leading role in the early stage, while the cooperation of both peptides takes over afterward. Our work demonstrates that it is normal to observe coacervates with different morphologies and functions due to kinetic control, especially in living cells. Peptides with minimized sequences are a practical approach to reveal the mechanism of Coacervation processes controlled by kinetics.
新设计多肽的动力学控制双相凝聚
通常把凝聚看作是液-液相分离过程,主要由热力学控制。然而,动力学可能起主导作用,特别是在包含多种相互作用的系统中。在这项工作中,我们使用(XXLY)6SSSGSS的肽来调整电荷密度和疏水性程度,以及引入二级结构,我们评估了动力学对在不同pH值下由单链寡核苷酸和季铵化葡聚糖组成的肽形成的双相凝聚的影响。只有在电荷密度恒定且静电相互作用是凝聚的主要驱动力的情况下,动力学的影响才可以忽略不计。当涉及ph依赖的静电相互作用和疏水相互作用或肽形成二级结构时,凝聚过程是路径依赖的,表明动力学控制了相分离过程。两种不同多肽结合的凝聚表明,具有较高电荷密度的肽在早期阶段起主导作用,随后两种多肽的合作起主导作用。我们的工作表明,由于动力学控制,特别是在活细胞中,观察到具有不同形态和功能的凝聚是正常的。具有最小序列的肽是揭示动力学控制的凝聚过程机制的一种实用方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


