Supramolecular Gelation Based on Native Amino Acid Tyrosine and Its Charge-Transfer Complex Formation
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
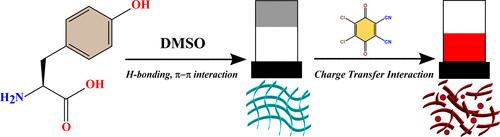
Self-assembly of amino acids and short-peptide derivatives attracted significant curiosity worldwide due to their unique self-assembly process and wide variety of applications. Amino acid is considered one of the important synthons in supramolecular chemistry. Self-assembly processes and applications of unfunctionalized native amino acids have been less reported in the literature. In this article, we are first-time reporting the self-assembly process of tyrosine (Tyr), an aromatic amino acid, in dimethyl sulfoxide (DMSO) solvent. Most of the studies related to Tyr self-assembly were reported in different aqueous solutions. In our work, we studied the self-assembly in several common organic solvents and found that Tyr could self-assemble into a supramolecular gel in dimethyl sulfoxide (DMSO) solvent. The self-assembly process was investigated by several techniques, such as UV–vis, fluorescence, FTIR, and NMR spectroscopy. Morphological features on the nanoscale were investigated through scanning electron microscopy (SEM). SEM images indicated the formation of nanofibrils with high aspect ratios. The supramolecular gel property was investigated by different rheological experiments. Computational study on the self-assembly process of Tyr in DMSO medium suggested that noncovalent interactions like hydrogen bonding and π–π stacking among the Tyr molecules played a prominent role. Finally, the charge-transfer complex formation ability of electron-rich Tyr with electron-deficient 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) was studied. In the presence of DDQ due to the charge-transfer complex formation, the supramolecular gel converted into a reddish color solution, and their fibrillar nanoscale morphologies collapsed.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


