Neural mechanisms of relational learning and fast knowledge reassembly in plastic neural networks
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
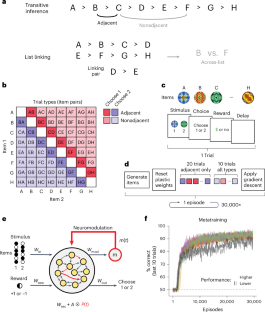
Humans and animals have a striking ability to learn relationships between items in experience (such as stimuli, objects and events), enabling structured generalization and rapid assimilation of new information. A fundamental type of such relational learning is order learning, which enables transitive inference (if A > B and B > C, then A > C) and list linking (A > B > C and D > E > F rapidly ‘reassembled’ into A > B > C > D > E > F upon learning C > D). Despite longstanding study, a neurobiologically plausible mechanism for transitive inference and rapid reassembly of order knowledge has remained elusive. Here we report that neural networks endowed with neuromodulated synaptic plasticity (allowing for self-directed learning) and identified through artificial metalearning (learning-to-learn) are able to perform both transitive inference and list linking and, further, express behavioral patterns widely observed in humans and animals. Crucially, only networks that adopt an ‘active’ solution, in which items from past trials are reinstated in neural activity in recoded form, are capable of list linking. These results identify fully neural mechanisms for relational learning, and highlight a method for discovering such mechanisms. Using a metalearning approach to model relational learning, Miconi and Kay show neural mechanisms for structured generalization and rapid learning.

塑性神经网络中关系学习和快速知识重组的神经机制
人类和动物在学习经验项目(如刺激、对象和事件)之间的关系方面有着惊人的能力,从而能够进行结构化归纳并快速吸收新信息。这种关系学习的一个基本类型就是顺序学习,它可以实现传递推理(如果 A > B 和 B > C,那么 A > C)和列表链接(A > B > C 和 D > E > F 在学习到 C > D 后迅速 "重新组合 "成 A > B > C > D > E > F)。尽管研究由来已久,但从神经生物学的角度来看,跨序推理和快速重新组合顺序知识的机制仍是个未知数。在这里,我们报告了具有神经调节突触可塑性(允许自主学习)并通过人工金属学习(learning-to-learn)识别的神经网络,能够执行反式推理和列表链接,并能进一步表达在人类和动物身上广泛观察到的行为模式。最重要的是,只有采用 "主动 "解决方案的网络才能够进行列表链接,在这种解决方案中,过去试验中的项目会以重新编码的形式重新出现在神经活动中。这些结果完全确定了关系学习的神经机制,并强调了发现这种机制的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


