Aromatic side-chain crosslinking in RiPP biosynthesis
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
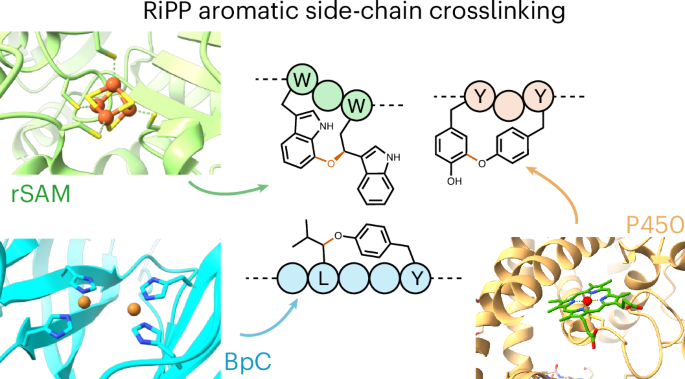
Peptide cyclization is a defining feature of many bioactive molecules, particularly in the ribosomally synthesized and post-translationally modified peptide (RiPP) family of natural products. Although enzymes responsible for N- to C-terminal macrocyclization, lanthipeptide formation or heterocycle installation have been well documented, a diverse array of cyclases have been discovered that perform crosslinking of aromatic side chains. These enzymes form either biaryl linkages between two aromatic amino acids or a crosslink between one aliphatic amino acid and one aromatic amino acid. Incredibly, nature has evolved multiple routes to install these crosslinks. While enzymes such as cytochromes P450 and radical S-adenosylmethionine (rSAM) enzymes are well known from other pathways, this role in RiPP biosynthesis has only recently been appreciated. Others, such as burpitide cyclases and DUF3328 (UstY) family proteins, come from eukaryotes and are relatively uncharacterized enzyme classes. This Review covers the emerging theme of aromatic amino acid side-chain crosslinking in RiPPs by focusing on the newly discovered enzymes responsible for catalyzing these challenging reactions. Aromatic side-chain crosslinking is emerging as a widespread and biosynthetically diverse feature in the RiPP natural products. This Review summarizes and unifies recent advancements, focusing on key enzymes that catalyze these modifications.

芳香侧链交联在RiPP生物合成中的应用
肽环化是许多生物活性分子的定义特征,特别是在核糖体合成和翻译后修饰肽(RiPP)家族的天然产物中。尽管对N-到c端大环化、硫肽形成或杂环安装的酶已经有很好的记录,但已经发现了一系列不同的环化酶,它们可以进行芳香侧链的交联。这些酶在两个芳香氨基酸之间形成联芳键,或在一个脂肪氨基酸和一个芳香氨基酸之间形成交联。令人难以置信的是,大自然已经进化出多种路径来安装这些交联。虽然细胞色素P450和自由基s -腺苷蛋氨酸(rSAM)酶等酶在其他途径中是众所周知的,但它们在RiPP生物合成中的作用直到最近才被认识到。其他的,如肽类环化酶和DUF3328 (UstY)家族蛋白,来自真核生物,是相对未被表征的酶类。本综述涵盖了RiPPs中芳香族氨基酸侧链交联的新兴主题,重点介绍了新发现的催化这些具有挑战性的反应的酶。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


