Design and Component Contribution Study of MIL-100(Fe)-Derived Materials for Adsorption Removal of Antipyrine from Water
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
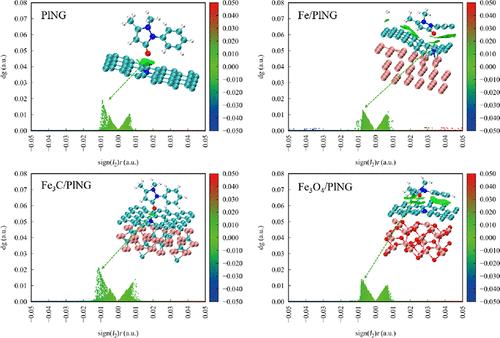
The use of metal–organic framework-derived materials for removing micropollutants from water is increasing, but precise composition control remains a challenge. In this study, 14 MIL-100(Fe)-derived materials were synthesized to adsorb and remove antipyrine (ANT) from water by optimizing the heating rate, pyrolysis temperature, and activation methods. The adsorbents demonstrated excellent ANT removal performance across a pH range of 3 to 11, as well as in complex water bodies. They also exhibited easy recovery and outstanding regeneration capabilities. Under the optimized conditions (i.e., ANT content: 10 mg/L, M-600–2 dosage: 0.1 g/L, and pH: 7), M-600-10 displayed the highest adsorption capacity (23.10 mg/g for ANT) while MIL-100(Fe) had limited adsorption capability (2.56 mg/g for ANT). The adsorption process was exothermic and spontaneous, influenced by both membrane diffusion and intraparticle diffusion, and followed the Freundlich and pseudo-second-order models. Despite variations in surface areas (75.70–261.91 m2/g), total pore volumes (0.2019–0.5984 cm3/g), and average pore sizes (7.47–13.99 nm), there was no strong linear relationship with adsorption capacity (7.47–15.99 mg/g). The major components, including Fe/pyrrolic N-doped graphene (PING), Fe3C/PING, Fe3O4/PING, and PING, had calculated adsorption energies of −0.94, −2.16, −0.79, and −2.24 eV, respectively, which correlated positively with Bader charge transfer numbers. The adsorbents relied on van der Waals forces for ANT adsorption, with PING exhibiting the strongest interactions and Fe3O4/PING exhibiting the weakest. These findings enhance the theoretical understanding of derived materials and expand their application in water purification.
MIL-100(Fe)衍生材料吸附去除水中安替比林的设计及组分贡献研究
金属有机框架衍生材料用于去除水中微污染物的使用正在增加,但精确的成分控制仍然是一个挑战。本研究通过优化加热速率、热解温度和活化方法,合成了14种MIL-100(Fe)衍生材料,用于吸附和去除水中的antipyrine (ANT)。在pH为3 ~ 11的范围内,以及在复杂的水体中,吸附剂表现出优异的ANT去除性能。它们还表现出易于恢复和出色的再生能力。在优化条件下(ANT含量为10 mg/L, M-600-2用量为0.1 g/L, pH为7),M-600-10对ANT的吸附量最高(23.10 mg/g), MIL-100(Fe)对ANT的吸附量有限(2.56 mg/g)。吸附过程为自发放热过程,同时受膜扩散和颗粒内扩散的影响,符合Freundlich和拟二阶模型。尽管比表面积(75.70 ~ 261.91 m2/g)、总孔隙体积(0.2019 ~ 0.5984 cm3/g)和平均孔径(7.47 ~ 13.99 nm)存在差异,但与吸附量(7.47 ~ 15.99 mg/g)之间没有很强的线性关系。主要成分,包括Fe/ pyrolic n掺杂石墨烯(PING)、Fe3C/PING、Fe3O4/PING和PING,计算出的吸附能分别为−0.94、−2.16、−0.79和−2.24 eV,与Bader电荷转移数呈正相关。这些吸附剂依靠范德华力吸附ANT,其中PING的相互作用最强,Fe3O4/PING的相互作用最弱。这些发现增强了对衍生材料的理论认识,扩大了衍生材料在水净化中的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


