Microwave-Activated Peroxyl Radicals Accelerate Hydroxymethyl Oxidation on the AuPd/C Catalyst for Mild Synthesis of FDCA
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
2,5-Furandicarboxylic acid (FDCA), an important platform chemical, is often synthesized from hydroxymethylfurfural (HMF), while the energy efficiency of the reaction is limited by the slow hydroxymethyl oxidation, resulting in poor technoeconomy. In this study, a bimetallic Au–Pd microwave-responsive catalyst was designed to achieve high-efficiency synthesis of FDCA by coupling microwave-activated peroxyl radical formation from hydrogen peroxide (H2O2). Pd sites were responsible for dissociating H2O2 into peroxyl radicals (·OOH), and Au sites accepted peroxyl radicals and oxidized HMF into FDCA. The microwave-boosted reaction synergy on the bimetallic Au–Pd dual active centers enabled the reaction to occur at near-room temperature (45 °C) and reached the highest FDCA yield of 88 mol % at 65 °C. Experimental and electric-field DFT studies revealed that microwaves increased the rate constant of H2O2 decomposition by 1.6-fold compared to conventional heating methods, with an ultrahigh H2O2 utilization rate of 90%. This work provides a new platform for highly efficient peroxyl radical formation with microwave energy, which can be extended to a wider range of oxidation reactions with accelerated reaction kinetics and enhanced energy efficiency.
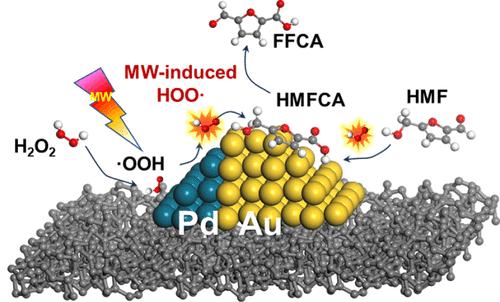
微波活化过氧自由基加速AuPd/C催化剂上羟基甲基氧化合成FDCA
2,5-呋喃二羧酸(FDCA)是一种重要的平台化学品,通常由羟甲基糠醛(HMF)合成,但由于羟甲基氧化缓慢,限制了反应的能量效率,导致技术经济性差。在本研究中,设计了一种双金属Au-Pd微波响应催化剂,通过微波激活过氧化氢(H2O2)形成过氧自由基,实现了FDCA的高效合成。Pd位点负责将H2O2解离成过氧自由基(·OOH), Au位点接受过氧自由基并将HMF氧化成FDCA。微波促进反应协同作用于双金属Au-Pd双活性中心,使反应在接近室温(45°C)下发生,并在65°C时达到88 mol %的FDCA产率。实验和电场DFT研究表明,微波对H2O2的分解速率常数比传统加热方式提高了1.6倍,H2O2的利用率高达90%。本研究为利用微波能量高效生成过氧自由基提供了一个新的平台,它可以扩展到更广泛的氧化反应中,加速反应动力学和提高能量效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
文献相关原料
公司名称
产品信息
阿拉丁
5-Hydroxymethylfurfural
阿拉丁
5-hydroxymethyl-2-furancarboxylic acid
阿拉丁
5-formyl-2-furancarboxylic acid
阿拉丁
2,5-furandicarboxylic acid
阿拉丁
5,5-dimethyl-1-pyrroline N-oxide
阿拉丁
O-diazophenanthrene complex indicator
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


