Exosomal miR-302b rejuvenates aging mice by reversing the proliferative arrest of senescent cells
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Cellular senescence, a hallmark of aging, involves a stable exit from the cell cycle. Senescent cells (SnCs) are closely associated with aging and aging-related disorders, making them potential targets for anti-aging interventions. In this study, we demonstrated that human embryonic stem cell-derived exosomes (hESC-Exos) reversed senescence by restoring the proliferative capacity of SnCs in vitro. In aging mice, hESC-Exos treatment remodeled the proliferative landscape of SnCs, leading to rejuvenation, as evidenced by extended lifespan, improved physical performance, and reduced aging markers. Ago2 Clip-seq analysis identified miR-302b enriched in hESC-Exos that specifically targeted the cell cycle inhibitors Cdkn1a and Ccng2. Furthermore, miR-302b treatment reversed the proliferative arrest of SnCs in vivo, resulting in rejuvenation without safety concerns over a 24-month observation period. These findings demonstrate that exosomal miR-302b has the potential to reverse cellular senescence, offering a promising approach to mitigate senescence-related pathologies and aging.
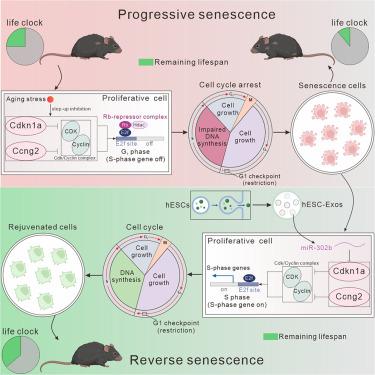
外泌体miR-302b通过逆转衰老细胞的增殖阻滞使衰老小鼠恢复活力
细胞衰老是衰老的一个标志,涉及细胞周期的稳定退出。衰老细胞(SnCs)与衰老和衰老相关疾病密切相关,使其成为抗衰老干预的潜在靶点。在这项研究中,我们证明了人类胚胎干细胞衍生的外泌体(hESC-Exos)通过恢复体外SnCs的增殖能力来逆转衰老。在衰老小鼠中,hESC-Exos治疗重塑了SnCs的增殖景观,导致了年轻化,这可以通过延长寿命、改善身体机能和减少衰老标志物来证明。Ago2 Clip-seq分析发现,在hESC-Exos中富集的miR-302b特异性靶向细胞周期抑制剂Cdkn1a和Ccng2。此外,在24个月的观察期内,miR-302b治疗逆转了体内SnCs的增殖停滞,导致返青而没有安全性问题。这些发现表明,外泌体miR-302b具有逆转细胞衰老的潜力,为减轻衰老相关病理和衰老提供了一种有希望的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
文献相关原料
公司名称
产品信息
索莱宝
SYBR Green PCR Master Mix
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


