Total synthesis of linear lipodepsipeptide kavaratamide A and its C25-epimer†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We report the stereoselective total synthesis of kavaratamide A, a linear lipodepsipeptide from the cyanobacterium Moorena bouillonii (collected in Kavaratti, India), and its unnatural C25-epimer. The convergent approach employs Keck asymmetric allylation to construct the chiral β-hydroxy carboxylic acid fragment [(3S)-HDA; 3-hydroxydecanoic acid], while the peptide unit was assembled from l-Val, N-Me-l-Ala, (S)-Hiva, and (S)-iPr-O-Me-pyr using well-orchestrated coupling methods to prevent racemization. Modifications to the Keck allylation conditions enabled the synthesis of the C25-epimer with good yield. Cytotoxicity of kavaratamide A and C25-epi-kavaratamide A, assessed using the MTT assay, demonstrated moderate activity against HepG2 and PANC-1 cell lines.
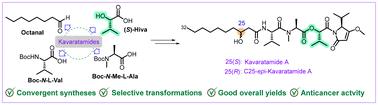
线性脂降肽kavaratamide A及其c25 -外显体的全合成。
我们报道了kavaratamide A的立体选择性全合成,kavaratamide A是一种线性脂沉肽,来自印度Kavaratti的morena bouillonii蓝藻(收集于Kavaratti, India)及其非天然c25 -外显体。收敛法采用Keck不对称烯丙化构建手性β-羟基羧酸片段[(3S)-HDA;3-羟基癸酸],而肽单元由L-Val、N-Me-L-Ala、(S)-Hiva和(S)-iPr-O-Me-pyr通过精心编排的偶联方法组装而成,以防止消旋。对Keck烯丙化条件的修改使c25 -外显体的合成具有良好的产率。使用MTT法评估kavaratamide A和C25-epi-kavaratamide A的细胞毒性,显示出对HepG2和PANC-1细胞系的中等活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


